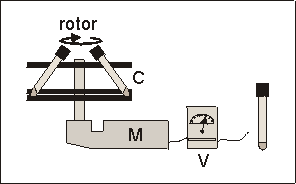
Fig. 6.1: Schematic presentation of the prototype centrifuge used for hypergravity experiments. Carousel (C), electric motor (M), variable voltage reducer (V). The culture tubes fitted into two plastic layers of the carousel at an angle of ± 55°.
Increased Mineralization and Calcium Release in Fetal Mouse Long Bones Cultured under Hypergravity Conditions.
Jack J.W.A. van Loon, J. Paul Veldhuijzen.
Academic Centre for Dentistry Amsterdam (ACTA), Dept. of Oral Cell Biology, Amsterdam, The Netherlands.
ABSTRACT
It is now well documented that skeletal tissues and cells are sensitive to their mechanical environment. It has been shown previously that fetal mouse metatarsal long bone rudiments respond with an increased mineralization and reduced mineral resorption when subjected to an intermittent (1/3 Hz) hydrostatic compression of 13 kPa above ambient, while in culture.(11,12) In the present study we applied gravitational forces, or accelerations, of 2.2g on bone rudiments for four to five days in organ culture, to study the effect of a vectorial force similar long on longitudinal growth and mineral metabolism. In the mineralization model, using 16 day old bones, 2.2g hypergravity stimulated cartilage growth by 28% and increased mineralization, as indicated by a more than 4 times longer calcified diaphysis as well as more than 7 times higher 45Ca incorporation. In addition, in the resorption model, using 17 day old bones, hypergravity increased mineral resorption by 37% compared to 1g controls. This data suggests that both growth and mineral metabolism in fetal bones are sensitive to mechanical stress resulting from hypergravity during organ culture.
Key words: mechanical loading, centrifugation, longitudinal growth, mineral metabolism.
6.1 INTRODUCTION
More than a century ago Wolff postulated his theory, better known as 'Wolff's law', which states that bone adapts its form and mass according to its loading history.(30) An in vivo sub-optimal loading history, as induced by immobilization(14,16) and spaceflight(18,19,34) is reflected by bone mineral loss (osteopenia). Higher than normal loadings, on the other hand, result in vivo(3) and in vitro(11) in an increased calcification. Application of mechanical stress to bone organ cultures allows to study responses in skeletal tissues at a more cellular level, without influence of systemic factors which may modulate or obscure the cellular response. In addition, in vitro models give the opportunity to control the magnitude, direction and frequency of the applied forces more easily.
One of the first in vitro studies on skeletal tissues in relation to mechanical forces have been performed by Glucksmann more then 50 years ago.(6) He used embryonic chick cartilaginous long bone rudiments to investigate tensile or compressive forces on rudiment development. One of his main findings was that skeletal tissues in vitro do respond directly to mechanical stresses. Later, various stresses like stretching substrata,(17,25) fluid shear,(20) and tissue compression(4,11) have been applied upon skeletal tissues and cells to reveal their responses and mechanisms to these mechanical loads. We have shown recently that in the, mechanically, nearly stressless environment of near weightlessness during spaceflight, mouse metatarsal long bones also react to the absence of a 1g acceleration.(28) However, very little is known about growth and mineral metabolism under increased accelerations.
In the present study we tested the hypothesis that hypergravity, applied to bone rudiments while in a tissue culture centrifuge, also affects mineral metabolism. The rudiments were cultured for four to five days in a prototype centrifuge generating a 2.2g mechanical stress. We used a mineralization as well as a mineral resorption model of 16 and 17 days old fetal mouse metatarsal long bones, respectively. Effects of matrix mineralization and mineral resorption as well as longitudinal growth of the whole bone were studied.
6.2 MATERIALS & METHODS
6.2.1 Mineralization model (16 day old metatarsals)
To study the effect of hypergravity on matrix mineralization, metatarsal long bones of 16 day old mouse fetuses (ED16) were used. The ED16 fetal mouse cartilaginous long bone rudiments are not yet mineralized at this stage of development. At this point the rudiments are still totally cartilaginous, and hypertrophic cartilage is seen in the central part of the rudiment in which matrix mineralization is initiated and which later constitutes the calcified diaphysis. The future bone collar around the hypertrophic center, is represented by a thin osteoid seam which is covered with a single layer of osteoblasts.
The rudiments were carefully, aseptically, dissected not disturbing the periosteum. ED16 metatarsals were cultured for four days in Alpha minimum essential medium without nucleosides (Gibco, Uxbridge, U.K.), supplemented with 10% heat inactivated normal rat serum (TNO, Rijswijk, The Netherlands), and 100 units/ml TC-penicillin / streptomycin (Difco, Detroit Mi, USA). In addition, 1.0 mM sodium--glycerophosphate (Sigma),(26) and 1 mCi 45CaCl2 /ml (specific activity 1.0 mCi/mmole; Radiochemical center, Amersham) was added only to the ED16 cultures. After culture the metatarsals were rinsed 3 times with Puck's saline G (Gibco) to remove free radioactivity. The incorporated 45Ca label was extracted in 1 ml 5% (v/v) formic acid over night at room temperature. The amount of incorporated 45Ca was determined by liquid scintillation counting.
6.2.2 Mineral resorption model (17 Day old metatarsals)
To study osteoclastic activity, metatarsal long bones of 17 day old mouse fetuses (ED17) were used. Sixteen days pregnant mice were injected, intraperitoneally, with 100 Ci 45CaCl2 (specific activity 1.0 mCi/mmole; Radiochemical center, Amersham. U. K.). During the following period this 45Ca is incorporated, in vivo, and labels all calcifying tissues including the mineralizing center (diaphysis) of the fetal metatarsals. The next day, 45Ca prelabeled rudiments were dissected and precultured at 37°C for one day to remove the freely exchangeable 45Ca. Then the medium was changed, and the ED17 rudiments were subsequently cultured for 5 days in total, with one medium change after three days. Subsequent culture conditions and medium were identical to that of the 16 day old metatarsals but no 45Ca and Na--glycerophosphate were added. Percentage release of 45Ca was calculated by counting the released 45Ca in the medium during culture, as well as measuring the remaining 45Ca in the rudiments after extraction (5% v/v formic acid). Radioactivity was measured using liquid scintillation counting.
For both mineralization and resorption studies, Swiss albino mice, inbred strain (University of Leiden, The Netherlands) were used.

Fig. 6.1: Schematic presentation of the prototype centrifuge used for hypergravity
experiments. Carousel (C), electric motor (M), variable voltage reducer (V).
The culture tubes fitted into two plastic layers of the carousel at an angle
of ± 55°.
6.2.3 Hypergravity culture
Fetal mouse long bones were cultured in glass tubes (volume 10 ml, PYREX) with a round bottom and air tight screw caps. Each tube contained 300 ml culture medium and one bone rudiment, and was flushed with a 5% CO2 in air gas mixture at the beginning of culture. Tubes remained closed during the subsequent culture period. To apply hypergravity, a prototype centrifuge was used (Fig. 6.1), which consisted of a carousel fitting a maximum of 8 experiment tubes. It was driven by an electric motor, which velocity could be varied by means of a variable voltage reducer to generate g-forces from 2.0g and higher. The tubes were held in cut-outs at a fixed angle of 55° (Fig. 6.1). With a radius at the tissue level of 125 mm and rotating at 118 rpm, this centrifugation resulted in a 2.2g acceleration. Control cultures (1.0g) were also placed at a 55° angle, to duplicate the centrifuge geometry. The centrifuged and control samples were all placed in the same 37°C incubator to avoid contingent temperature, electromagnetic and vibration effects.
6.2.4 Morphometry
Total length of the metatarsals and length of the diaphysis (mineralized zone) were measured with a eyepiece graticule fitted into a Nikon stereo microscope at a final magnification of 40. Lengths were measured at the beginning of the final culture period, and again after 4 (ED16) or 5 days (ED17). %Length increase was determined as length (Tend-Tbegin)/Tbegin100%.
6.2.5 Statistics
To reduce sample variations metatarsals were contralateral paired: controls (1g) with treated (2.2g). Statistics were calculated using the two tailed Student t-test for paired observations for data in Table 6.I and Figs. 6.2 and 6.3. A Student t-test ANOVA for paired observations was applied to the data in Fig. 6.4. For these calculations the g-force was entered as a in between subject variance. All data are expressed as mean ± SEM.
6.3 RESULTS
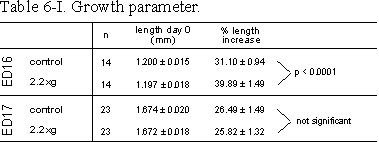
Table 6.I: Growth parameter. Lengths of ED16 and ED17 metatarsals before and
after 4 days of culture at control or 2.2g conditions. Rudiments were
contralateral paired. %Length increase is determined as length (T4-T0)/T0100%.
Data are expressed as means ± SEM.
All metatarsals cultured under 2.2g acceleration showed a normal morphological appearance, compared to the control cultures, as observed with a stereo microscope during and after culture.
In the mineralization model, ED16 bones, longitudinal growth was significantly increased by 2.2g (Table 6.I). During the four day culture period total length of ED16 metatarsals increased from 1.20 to 1.57 mm for control and from 1.20 to 1.67 mm for 2.2g cultures. The increase of the total length in the 2.2g group was more than 25% larger compared to controls (Table 6.I).
Mineralization, as measured by the length of the diaphysis in ED16 metatarsals, started during culture under unit gravity conditions. Culture under 2.2g acceleration resulted in a strong increase of 468% in length of the diaphysis compared to the 1g controls (Fig. 6.2). Compared to controls there was also a 751% increase in radioactive calcium content of the mineralizing cartilage and the primitive bone collar of the 2.2g group (Fig. 6.3).
In the ED17 resorption model, general growth of the whole rudiments was not changed by 2.2g (Table 6.I). Mineral resorption, as measured by cumulative 45Ca release into the medium, increased under 2.2g conditions, by 26 and 37% at 2.2g, after 3 and 5 days culture, respectively (Fig. 6.4).
6.4 DISCUSSION
In the present in vitro experiments we found that increased gravity, modulated longitudinal growth, matrix mineralization and mineral resorption in fetal mouse cartilaginous long bone rudiments.
The stress used for this study, gravity, is a vectorial force, determined by magnitude and direction. The orientation of the gravity vector acting on the long bones during culture was not longitudinal but perpendicular with respect to the bones' long axis. In vivo loading of weight bearing long bones is certainly not always in the longitudinal direction. However, at the cellular level the direction of the force is probably less important for cellular behavior.(2) Only at a higher level of organization are stress directions important for the anatomical function of bone.
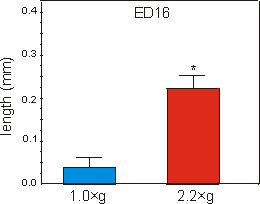
Fig. 6.2: Lengths of the mineralized zone (diaphysis) of ED16 metatarsal long
bones cultured for four days under 1g control, or at 2.2g hypergravity
conditions. Data are expressed as means ± SEM, n=14, *p<0.0005.
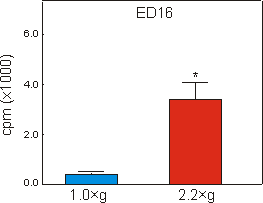
Fig. 6.3: 45Calcium content of the mineralizing diaphysis of ED16
metatarsal long bones, cultured for four days under 1g control, or 2.2g
hypergravity conditions. Data are expressed as means ± SEM, n=16, *p<0.005.
In an earlier study, using similar bone rudiments, mineralization was also accelerated, by applying intermittent hydrostatic force (ICF) to the rudiments.(11) Under hydrostatic pressure the force is omni-directional, while the present experimental condition produced an uni-directional force (see also Fig.6.5). However, Wong and Carter(31) have shown that the accelerating effect of ICF probably resulted from shear force generated at the mineralized non-mineralized interface in the diaphysis, and not from hydrostatic pressure per se. Hypergravity also produces shear stress at similar interfaces between heavy, mineralized tissue and lighter non-mineralized tissue. Therefore it is possible that both treatments. ICF and hypergravity, resulted in a similar effect at the front of mineralization, which explains their common effect. A detailed finite element analysis of both mechanical situations is needed to substantiate this suggestion.
In pilot experiments, using a slightly different tissue culture centrifuge (courtesy Dr. J. Duke, Houston, USA), a 3.1g force had a still higher effect on mineralization in ED16 bones than had a 2.5g load (data not shown). This indicates that the mechanoreceptor system of these metatarsals was still not saturated at a 2.5g acceleration. A dose dependent effect of gravity forces was also suggested in a study by Inoue et al.(10) They reported a dose dependent increase in DNA thymidine uptake in mouse osteoblasts like MC-3T3 cells cultured under various accelerations. Such a dose dependent stress sensitivity is also in accordance with in vivo studies by Rubin and Lanyon.(22) They showed that peak strains, applied to mechanically isolated turkey ulnae, resulted in a graded dose:response change in bone mass.
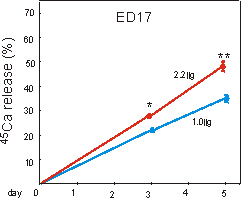
Fig. 6.4: Percentage 45calcium release from prelabeled ED17 metatarsal
long bones, cultured for five days under 1g control, or 2.2g
hypergravity conditions. Data are expressed as means ± SEM, n=30, *,**p<0.005.
Besides experiments with single cells or tissues, several studies have been performed with the centrifugation of whole animals. In various studies running for 14 days up to three generation times, rodents were kept at various accelerations, ranging from 1.1g to 3.0g.(23,24,33) Some general phenomena noted were decreased body weights and decreased lengths of the femora and other long bones. It is important to realize, however, that in these studies elevated levels of systemic stress hormones and factors may have been responsible for the observed effects. Glucocorticoids, as a result of psychological stress for example, are known to change bone metabolic processes.
For reasons unknown, the enhanced growth of the ED16 2.2g samples compared to controls, was not seen in ED17 bones. The only differences between the ED16 and ED17 cultures were the stage of development and the addition of 1 mM of Na--glycerophosphate to ED16 medium. This suggests that longitudinal growth of younger ED16 metatarsals is more sensitive to a increased gravitational load compared to one day older, ED17 bones. It could also be that the addition of GP to the ED16 cultures sensitized the bones responsiveness to 2.2g.
In the present study mechanical loading by 2.2g increased mineral resorption as measured by 45Ca release. This is in contrast to earlier studies where intermittent hydrostatic pressure inhibited resorption.(12) An important difference between the present study and the experiments with intermittent loading is the continuous application of stress during centrifugation. In vivo experiments by Lanyon et al. have shown that, in turkeys ulnas, intermittent application of loads resulted in increased bone mass while the continuous application of the same loads reduced bone mass compared to controls.(14) Also, tissue culture experiments using embryonic chicken epiphyseal cartilage, cultured under continuous pressure showed decrease in cyclic AMP accumulation,(21) while intermittent application of a similar force resulted in an increased cAMP production.(29) This suggests that the intermittent application of mechanical stress may lead to different responses compared to static loads. Static load experiments were also performed by Gazit.(5) She also reported an increase in Ca2+ release from mouse calvariae cultured under continuous increased gravity of 1.9g. It was argued that the increased acceleration caused an increase in static pressure, which might be responsible for the increased resorption in these calvarial explants. Dynamic versus static hypergravity experiments can now be performed by using a newly developed computer controlled centrifuge.(27) With this tissue culture centrifuge it is possible to apply various hypergravity forces onto cells and tissues either intermittently or statically, ranging from 1.0 to 100g. With this apparatus the hypothesis as discussed above should be tested in the near future.
The two methods for monitoring mineralization, optically by length of the diaphysis and biochemically, by 45Ca content produced somewhat different results. Compared to the control (1.0g) cultures the 2.2g group had a 468% increase in length of the mineralized zone while a 751% increase was found in 45Ca uptake (Figs. 6.2 and 6.3). As the optical measurements are two dimensional, while the biochemical data originate from a three dimensional volume, these differences may derive from the formation of a more dense diaphysis as a whole. Since the diameter of the mid diaphyseal area in the bones did not change significantly during culture (data not shown), wider trabeculae or denser mineral may have been formed in the loaded metatarsals. Histomorphometrical studies might clarify this discrepancy.
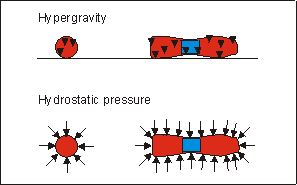
Fig. 6.5: Schematic representation of direction and application site of forces
in hypergravity and hydrostatic pressure(Klein-Nulend et al.
1986) experiments in metatarsal long bones.
To date it is not clear what the cellular mechanoreceptor and its direct down stream intermediates in skeletal tissues are. In non-skeletal HeLa cells, increased 3H-thymidine incorporation has been reported when cells were cultured at 18, 35 and 70g.(13) This increased proliferation occurred in parallel with an increased c-myc mRNA expression. EGF-induced c-fos expression in human A431 epidermoid carcinoma cells cultured at 10g was enhanced, while in simulated(8) and real(7) microgravity this expression was decreased, compared to 1g controls. It has also been shown that c-fos, and c-myc, are associated with chondrocyte proliferation and differentiation in cultures from mouse mandibular condyles.(1) A transient expression of c-fos in condyle cells was argued to be associated with mechanical stimulation.(1) It may be that, on a cellular level, hypergravity also acts via a modulation of proto-oncogene expression which regulates growth and differentiation, thereby changing mineral metabolism. Future experiments should therefore focus on changes in c-fos and c-myc proto-oncogene levels, as well as related second messenger molecules and signal transduction pathways.
Summarizing, mineralizing ED16 and resorbing ED17 fetal mouse metatarsal long bones were shown to react to increased gravitational forces of 2.2g. Hypergravity had a stimulating effect on growth and mineralization and increased mineral resorption. We showed earlier that these fetal long bones also respond to the nearly absence gravity during spaceflight.(28) Therefore these fetal bone organ cultures are useful models to further study the skeletal metabolism under hypogravity and hypergravity conditions.
6.5 REFERENCES
1 Closs E.I., Murray A.B., Schmidt J., Schon A., Erfle V., Strauss P.G. c-fos Expression precedes osteogenic differentiation of cartilage cells in vitro. J. Cell Biol. III, 1313-1323, 1990.
2 Cowin S.C., Moss-Salentijn L., Moss M.L. Candidates for the mechanosensory system in bone. BED Vol 20, Adv. in Bioengineering, ASME, 313-316, 1991.
3 Dalsky G. P. Socke K.S., Ehsani A.A., Slatopolsky E., Lee W.C., Birge S.J. Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann. Intern. Med. 108, 824-828, 1988.
4 El Haj A.J., Minter S.L., Rawlingson S.C.F., Suswillo R., Lanyon L.E. Cellular responses to mechanical loading in vitro. J. Bone Min. Res. 5, 923-932, 1990.
5 Gazit E. Effect of increased gravity on bone resorption in the mouse calvaria explant system. Isr. J. Med. Sci. 16, 867-869, 1980.
6 Glucksmann A. Studies on bone mechanics in vitro. II. The role of tension and pressure in chondrogenesis. Anat. Rec. 73, 39-56, 1942.
7 Groot de R.P., Rijken P.J., Hertog den J., Boonstra J., Verkleij A.J., Laat de S.W., Kruijer W. Microgravity decreases c-fos induction and serum response element activity. J. Cell Sci. 97, 33-38, 1990.
8 Groot de R.P., Rijken P.J., Boonstra J., Verkleij A.J., Laat de S.W., Kruijer W. Epidermal growth factor induced expression of c-fos is influenced by altered gravity conditions. Aviat. Space Environ. Med. 62, 37-40, 1991.
9 Huiskes R., van Donkelaar C.C., Jepsen K.J., Weinans H., Goldstein S.A., Burger E.H. The mechanical consequences of mineralization in fetal bone. Abstract of the 41st annual meeting of the Orthopaedic Research Society, Febr. 13-16, 1995, Orlando FL, U.S.A. (accepted).
10 Inoue H., Hiasa K., Samma Y., Nakamura O., Sakuda M., Iwamoto M., Suzuki F., Kato Y. Stimulation of proteoglycan and DNA syntheses in chondrocytes by centrifugation. J. Dent. Res. 69, 1560-1563, 1990.
11 Klein-Nulend J., Veldhuijzen J.P., Burger E.H. Increased calcification of growth plate cartilage as a result of compressive force in vitro. Arthritis Rheum. 29, 1-9, 1986.
12 Klein-Nulend J., Veldhuijzen J.P., Strien M.E. van, Jong M. de, Burger E.H. Inhibition of osteoclast bone resorption by mechanical stimulation in vitro. Arthritis Rheum. 33, 66-72, 1990.
13 Kumei Y., Nakajima T., Sato A., Kamata N., Enomoto S. Reduction of G1 phase duration and enhancement of c-myc gene expression in HeLa cells at hypergravity. J. Cell Sci. 93, 221-226, 1989.
14 Lanyon L.E. Functional strain as a determinant for bone remodeling. Kroc Foundation Conference on Functional Adaptation in Bone. Calcif. Tissue Int. 36, 556-561, 1984a.
15 Lanyon L.E. and Rubin C.T. Static vs dynamic loads as an influence on bone remodelling. J. Biomechanics 17, 897- 905, 1984b.
16 LeBlanc A.D., Schneider V.S., Evans H.J., Engelbretson D.A., Krebs J.M. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Min. Res. 5(8), 843-850, 1990.
17 McDonald F., Houston W.J.B. The effect of mechanical deformation on the distribution of potassium ions across the cell membrane of sutural cells. Calcif. Tissue Int. 50, 547-552, 1992.
18 Morey E.R., Baylink D.J. Inhibition of bone formation during space flight, Science 201, 1138-1141, 1978.
19 Parfitt A.M. Bone effects of space flight: Analysis by quantum concept of bone remodelling. Acta Astron. 8(9-10), 1083-1090, 1981.
20 Reich K.M., Frangos J.A. Effect of flow on prostaglandin E2 and inositol triphosphate levels in osteoblasts. Am. J. Physiol. 261, C428-C432, 1991.
21 Rodan G.A., Bourret L.A., Harvey A., Mensi T. Cyclic AMP and cyclic GMP: mediators of the mechanical effects on bone remodeling. Science 467-469, Aug. 8, 1975.
22 Rubin T.R., Lanyon L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 37, 411-417, 1985.
23 Simon M.R., Holmes K.R., Olsen A.M. The effects of quantified amounts of increased intermittent compressive forces for 30 and 60 days on the growth of limb bones in the rat. Acta Anat. 120, 173-179, 1984.
24 Smith S.D. Effects of long-term rotation and hypergravity on developing rat femurs. Aviat. Space Environ. Med. 46(3), 248-253, 1975.
25 Somjen D., Binderman I., Berger E., Harell A. Bone remodelling induced by physical stress is prostaglandin E2 mediated. Biochimica et Biophysica Acta 627, 91-100, 1980.
26 Tenenbaum H.C. Role of organic phosphate in mineralization of bone in vitro. J. Dent. Res. 60 (C), 1506-1509, 1981.
27 van Loon J.J.W.A., van den Bergh L.C., Schelling R., Veldhijzen J.P., Huijser R.H. 1993 Development of a centrifuge for acceleration research in cell and developmental biology. 44th Intern. Astron. Congr., IAF/IAA-93-G.4-166, 39, Graz, Austria, 16-22 October 1993.
28 van Loon J.J.W.A., Bervoets D.J., Burger E.H., Dieudonné C.S., Hagen J.W., Semeins C.M., Zandieh Doulabi B., Veldhuijzen J.P. Decreased mineralization and increased calcium release in isolated fetal mouse long bones under near weightlessness. J. Bone Min. Res., accepted for publication in April 1995.
29 Veldhuijzen J.P., Bourret L.A., Rodan G.A. In vitro studies of the effect of intermittent compressive forces on cartilage cell proliferation. J. Cell. Physiol. 98, 299-306, 1979.
30 Wolff J.D. Das Gezetz der Transformation der Knochen, Berlin, A. Hirschwald, 1892.
31 Wong M., Carter D.R. Theoretical stress analysis of organ culture osteogenesis. Bone 11, 127-131, 1990
32 Wunder C.C., Cook K.M., Welch R.C., Glade R., Fleming B.P. Femur bending properties as influenced by gravity: Ultimate load and moment for 3-G rats. Aviat. Space Environ. Med. 48, 339-346, 1977.
33 Wunder C.C., Cook K.M., Watkins S.R., Moressi W.J. Femur bending properties as influenced by gravity: V. Strength vs. calcium and gravity in rats exposed for 2 weeks. Aviat. Space Environ. Med. 58, 977-82, 1987.
34 Yagodovsky V.S., Triftanidi L.A., Gorokhova G.P. Space flight effects on skeletal bones of rats (Light and electron microscopic examiniation). Aviat. Space Environ. Med. 47, 734-738, 1976.
6.6 ACKNOWLEDGMENTS
The authors like to thank Dr. P.J. Duke, University of Texas, Dental Science Institute, Houston, U.S.A. for using their tissue culture centrifuge for pilot experiments. Dr. D.R. Carter, Stanford University, Stanford, USA; Dr. ir R. Huiskes, and ir W. van Driel, University of Nijmegen, Nijmegen, The Netherlands for their helpful suggestions on parts of the manuscript.
This study was supported by a grant (MG-004) from The Dutch Organization for Scientific Research (NWO) through the Space Research Organization of the Netherlands (SRON).