1.1 Bone and Spaceflight: An Overview.
Jack J.W.A. van Loon
Academic Centre for Dentistry Amsterdam (ACTA), Dept. of Oral
Cell Biology, Free University, Amsterdam, The Netherlands.
With
minor changes published
in: Jack J.W.A. van Loon, J. Paul Veldhuijzen, Elizabeth H. Burger. Bone and
space flight: an overview. in Biological and Medical Research in Space, Edt.
D. Moore, P. Bie and H. Oser. Springer-Verlag Berlin Heidelberg, Chapter 5,
259-299, 1996.
Some four centuries ago it was already recognized by Galileo
Galilei (1564-1642) that the rigidity of the skeleton of terrestrial animals
is related to its load bearing function, which is associated with the animals'
size and mass(21)(see Fig. 1.1.).
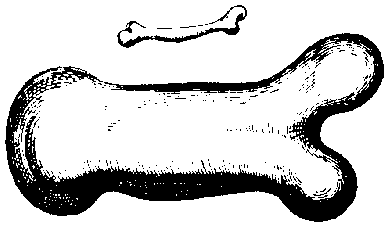
Fig. 1.1. A drawing by the Italian Galileo Galilei (1564-1642) demonstrating
the dimensions of the bones from animals of distinct weights. It is obvious
that the length-to-width ratio is remarkably different between light and heavy
animals. (After G. Galilei, Two new Sciences, translated by Stillman Drake,
The University of Wisconsin Press, 1974.)
This phenomenon of differences in mechanical properties also
pertains to the relatively small variations in the skeleton which occur between
individuals of the same species, as well as to variations in bone properties
within the same subject. Ward(84) observed that the trabecular arrangement
within the femoral head, the area now known as Ward's triangle, shows patterns
comparable to those found in the crossbeam structures of nineteenth century
streetlights. This very clearly illustrates natures' way of mechanically engineering
the weight bearing properties of bone. Nearly 50 years later Wolff postulated
his 'law'; 'Das Gezetz der Transformation der Knochen'.(89) In this
essay he postulated in more detail that the structure of bone is reflecting
its mechanical usage history. The process of bone formation and bone remodeling
according to its mechanical history is now generally known as Functional Adaptation,
a term proposed by Roux.(58)
Since the late nineteenth century, many studies have been performed
to evaluate this phenomenon of functional adaptation of the skeleton. It has
been shown that in people with a sedentary lifestyle the average bone loss is
more prominent than in more ambulatory subjects. This demonstrates that not
using the skeleton, i.e. not applying loads onto the bones, leads to
mechanical inferiority. This loss can be measured as a reduction in bone mineral
density, a reduction in trabecular structure or changes in the biochemical composition
or arrangement of the organic matrix components. On the other hand, by exceeding
the normal loads by weight bearing exercise,(55,56) or running(1,11,94)
bone mass can increase compared to control or pretreatment conditions.
Bone loss or gain is related to the magnitude, direction and
frequency of the stress acting upon the skeleton while applying loads. The resulting
strain (e) is defined as a dimensionless measure for linear deformation, one
strain being a 100% deformation in length of a piece of material after applying
a load. No such high values are found in bone biology, where deformations lie
in the range of several hundreds of micro-strains (me). Depending on the final
shape change of the material, three forms of strains are identified, namely
tensile, compressive and shear strains. In all materials, also bone, stresses
are linearly related to the applied loads. The amount of strain, however, is
related to the mechanical properties of the material, the Young's modulus, and
does not have to be a linear function of stress.
From various in vivo studies(35) is has become
clear that strains from 1,500-3,000 me promote bone modeling while strains beneath
these values correspond to increased remodeling activity in bone.(20)
However, strain values exceeding 6,000 me lead to bone fatigue and final fractures.(60)
So it is the height of the peak strain which renders an anabolic effect on bone
mass,(59) but also the frequency of application may play an important
role.(61)
In a situation of free floating in a spacecraft there is no
weight bearing function of the skeleton, i.e,. there is no weight bearing
stress. This means that, through the initiation of orbital spaceflight, in the
late nineteen fifties, an environment has been introduced providing very low
strains. Since then, several studies have indicated that spaceflight poses various
detrimental effect upon the human body, as a result of near weightlessness or
as a result of accumulating radiation outside the shielding Earth atmosphere.
Affected are the neurovestibular functions, shift of various body fluids, cardiovascular
function, various hemopoietic parameters and the musculo-skeletal system (see
Fig. 1.2).(45)
The skeleton has evolved during millions of years in unit gravity
on Earth, but in a near weightless, or microgravity, state in space there does
not seem to be much need for such a rigid weight bearing structure. Based on
data of skeleton unloading studies, it seems likely that there may be signs
of bone loss after being in a near weightlessness condition in space. Although
most of the changes in physiological parameters found under spaceflight conditions
reach a level of adaptation at about 6 weeks or sooner (see Fig. 1.2), it is
still obscure what the adaptation time and mechanical stability for bone will
be after long periods of microgravity.
The relatively new era of research on vertebral life probing
extraterrestrial space started with the launch of the dog Layka, November third
1957 in the Sputnik-2, followed nearly four years later, on April 12 1961, by
the first human being, Yoeri Gagarin.
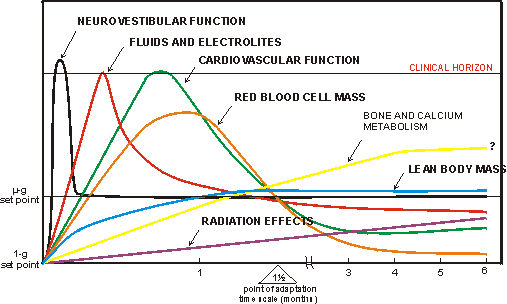
Fig. 1.2. A schematic presentation of the main changes occurring in a human
body in the near weightlessness environment of space. 'The 1´g set point represents
physiological status on Earth. mg set point denotes a complete physiological
adaptation level in space which probably can only be achieved by individuals
born in space. Point of adaptation is the average time of 6 weeks for a visitor
to space to exhibit partial adaptation to the environment.' (After Nicogossian
A.E. In: Space Physiology and Medicine, Lea & Febiger, 1989.)
In the following we will try to summarize data about in
vivo bone growth and metabolism which has been accumulated since the start
of spaceflight. We have tried to be as complete as possible, using original
papers published in, mostly Western, peer reviewed literature. Less attention
is given to the Russian, or former Soviet, literature merely due to the language
barrier, although some data from Russian investigators is included. One should
keep in mind in this respect, that the Russians have far more experience both
in number of flights and total flight hours in space than all other nations
combined.(8)
The main spaceflights covering a period of nearly 30 years
involving in vivo bone research are tabulated in, mostly, chronological
order in Table 1.I. The 'recovery time' listed in this table is actual time
between the landing of the space craft and the post flight handling of the samples.
Table 1.II provides more detailed information about the particular
flights involved. In the column 'species' in this table, only the species involved
in bone research are listed, this is also true for the adjacent column, 'number
of individuals'. The number indicated is based on the maximum number of animals
used for at least one of the studies reviewed in this paper. The total number
of samples used by other studies in the same flight may vary from the number
indicated in this column. During Cosmos 605 e.g., 10 rats and two boxes
filled with amphibians and reptiles were on board. For this particular mission
only 5 rats were used for bone related studies.(93)
Five main groups of flights can be distinguished in this overview.
The Gemini flights, the long duration Skylab missions, the Russian Cosmos series,
the very long duration Salyut-7 and Mir missions, and the relatively short flights
of US Space Shuttle.
More than four years after the first man in space, the Gemini
missions were launched. One of the first studies on bone loss in relation to
spaceflight emerged from this flight, and was reported by Mack et al.(38),
later corrected by Vose.(83) They reported a reduction in bone density
during these flights and showed that mineral loss in the os calcis was 2.9-9.2%
after 4-14 days of orbital spaceflight. Metabolic studies have also been performed
during these flights. Lutwak et al.(37) revealed a clear trend
towards an increased urinary calcium excretion in the Gemini-VII flight. In
one individual this decrease also persisted during a four days post flight period.
Also urinary phosphate excretion increased in flight in both Gemini astronauts.(37)
In the Biosat-III satellite non-human primate (Macaca
Nemestrina) study, a ground control monkey was included which stayed in
the same hardware as the flight animal. In these primates an average of bone
mineral density loss of 4.5% at various anatomical sites was reported after
a nearly 9 days flight.(39)
Few reports on life sciences experiments in the Apollo
series of spaceflights have been published in peer reviewed papers. During Apollo-XVII,
a metabolic study on calcium and phosphorus metabolism has been reported by
Rambaut et al.(57) For this study, astronauts scored all their
diet intakes and fecal and urinary excretions. A reduction of about 0.2% in
total body calcium and 0.7% in total body phosphorus was found after this 14
days Apollo flight. These losses were mainly due to an increased fecal calcium
and an increased urinary and fecal phosphorus excretion while under near weightlessness.
The losses were comparable to reductions reported in bed rest studies.(57)
The authors argued that the changes found may be attributed not only to hypogravity
or immobilization but also to disturbances in gastrointestinal absorption. However,
other metabolic studies have been performed all indicating increased calcium
excretion.(30,37,87) In the same study by Rambaut et al. the
authors also reported a small but significant loss of total body weight in the
Apollo-XVII crew.(57) This has been confirmed during the 28 days
Skylab-II mission.(87) For Apollo flights this could have been caused
by the near weightlessness period in space, by immobilization while being strapped
into the modules' seats, or by the relative hypogravity on the lunar surface
and / or other systemic influences. For Skylab flights, however, probably only
near weightlessness related phenomena should have caused bone loss, since there
was ample space to move about in the large Skylab modules. Reduced bone mineral
content in Apollo crew was also reported by Vogel et al.(81)
Besides two fishes (Fundulus) in the Skylab-III
mission, there were no vertebrate animals on board these semi-long duration
flights. In analogy to the Gemini experiment, bone mineral content (BMC) measurements
were performed on the Skylab crew before and after the mission. Vogel et
al. reported a negative trend towards a decreased BMC, especially in the
os calcis and radius.(82) In two crew members of the Skylab-IV mission,
this bone loss had not returned to baseline values even after 97 days post flight.
The calcium balance in Skylab-II crew was determined in an
extensive study by Whedon et al.(87) A mean pre flight value
of this balance of +71 mg/day was recorded, while the mean balance during the
last 16 days in flight was -50 mg/day, a negative shift of 121 mg/day. Increased
phosphorus and nitrogen excretion was also noted, indicating a loss of soft
tissues, probably muscles.(87)
In the mid nineteen seventies a Cosmos series of spaceflights
was initiated. These flights were a collaboration of the Soviets with various
other nations including Czechoslovakia, France, Hungary, Poland, Rumania and
the United States of America. These unmanned missions, sometimes also referred
to as Biocosmos, involved mainly life sciences experiments. At that time these
satellites were the only completely automated space crafts, and they were capable
of staying in orbit for nearly three weeks, while maintaining normal ambient
environmental conditions. They could be retrieved and the biological samples
recovered for on ground analysis. Much of the early, more detailed, bone studies
derive from these flights.(42,74,93) The rat was the main species
involved, although, especially in the later missions, non-human primates were
also part of the payload. Results of studies on bone and Rhesus monkeys under
near weightlessness have been reported for Cosmos 1877 and 2044 flights by Zerath
et al.(95,96)
The experimental setup for the Russian Cosmos program is quite
consistent over the years. Basically there are four groups:
1: Basal (B). This group of animals is killed at the start
of the mission, and gives a baseline value for the various parameters investigated.
Especially with young rapidly growing rats this group is important to distinguish
between growth and spaceflight-induced effects.
2: Flight (F). This group actually flies on board the spacecraft. Several days
before launch the animals are transferred from standard laboratory cages into
the special spaceflight hardware, cylindrical cages of 20.5 cm long and 9.4
cm in diameter. Housed in this hardware they are fed pasty diets and water ad
lib. During launch this group is subjected to various g-forces, vibrations
and noise levels. While in orbit there is a regular light-dark cycle (± 2 lux
intensity) and normal room temperatures.
3: Synchronous (S). As the term indicates, this group is synchronized as close
as possible with the actual flight group. This means that the animals follow
the same procedure as used for flight. One important element is that the launch
characteristics experienced by the flight group are simulated for the synchronous
animals. Noise levels of up to 110 db and vibrations of 50-70 Hz at an amplitude
of 0.4 mm for 10 minutes are imposed upon the animal holding units. After this,
the animals are subjected to a series of accelerations for 10 minutes with a
plateau at 4´g for 7 minutes to simulate take-off. Re-entry loads, while returning
to Earth, are simulated by applying g-forces for 6 minutes with a 6´g plateau
for 3 minutes. The actual impact shock at touch down is 50´g for 10 msec.(69)
Table 1.I. Flights covered in this bone studies overview.
|
Flight number: |
Launch date: |
Flight duration: day:hour:minute |
Recovery time: |
|
Gemini-4 |
3 March 1965 |
04:01:58 |
? |
|
Gemini-5 |
21 August 1965 |
07:22:59 |
? |
|
Gemini-7 |
4 December 1965 |
13:18:35 |
? |
|
Gemini-6 |
15 December 1965 |
01:01:51 |
? |
|
|
|
|
|
|
Biosatellite-III |
June 1969 |
08:20:-- |
? |
|
|
|
|
|
|
Apollo-17 |
7 December 1972 |
12:13:51 |
? |
|
|
|
|
|
|
SkyLab-II |
25 May 1973 |
28:00:50 |
? |
|
SkyLab-III |
28 July 1973 |
59:11:09 |
? |
|
SkyLab-IV |
4 December 1973 |
84:01:16 |
? |
|
|
|
|
|
|
Cosmos 605 |
October 1973 |
22:--:-- |
2 days |
|
Cosmos 782 |
25 November 1975 |
19:12:-- |
3 days |
|
Cosmos 936 |
3 August 1977 |
18:12:-- |
? |
|
Cosmos 1129 |
25 September 1979 |
18:12:-- |
7-11 hrs |
|
Cosmos 1514 |
14 December 1983 |
05:00:-- |
< 6 hrs |
|
Cosmos 1667 |
10 July 1985 |
06:12:-- |
< 6 hrs |
|
Cosmos 1887 |
29 September 1987 |
12:12:-- |
42 hrs |
|
Cosmos 2044 |
15 September 1989 |
14:00:-- |
6-10 hrs: monkeys 11-34 days |
|
Salyut-7 |
1982 |
years |
? |
|
|
|
|
|
|
SpaceLab-3 (SL-3)
(STS-51B) |
26 April 1985 |
07:00:09 |
Flight: R+11 hrs: Sim: R+60 hrs |
|
SpaceLab-2 (SL-2)
(STS-51F) |
29 July 1985 |
07:22:46 |
? |
|
|
|
|
|
|
Mir |
Februeri 1986 |
years |
? |
|
|
|
|
|
|
Space Shuttle (STS-41) |
6 October 1990 |
04:02:10 |
? |
|
Space Shuttle (STS-52) |
22 October 1992 |
09:20:56 |
? |
L = launch, R = recovery, STS= Space Transport System (= US Space Shuttle)
The synchronous group normally lags behind the flight group.
This is to provide time to adjust the ground simulation setup according to actual
flight data input. Unexpected changes in e.g. temperatures in the flight
module can be programmed into the ground module to duplicate the orbital status
as close as possible. The differences between the flight and the synchronous
group are of course the near weightlessness condition but also the galactic
radiation experienced by the flight group and the difference in start time of
the experiment. The effects of spaceflight, or microgravity, on the various
parameters are therefore at best compared to the synchronous group. The ultimate
control group, however, housed in an on board 1´g centrifuge, was used only
twice, during the Cosmos 782 and Cosmos 936 flights. These centrifuges had a
radius of 32 cm and held rats which were individually housed in cages of 9.4
´ 20.5 cm.
4: Vivarium (V). The vivarium group derives from the same pool of animals as the former three groups. This group is not housed in the special, rather small, flight cages, but in standard laboratory cages, which leaves them more space and greater freedom. This group is not subjected to any launch related phenomena.
Cosmos 605 was the first in these series of Cosmos flights. After the 22 day mission, Yagodovsky et al. found signs of osteopenia in rat long bones.(93) In their microscopical study they also reported an increase in the width of osteocyte lacunae in flight samples which suggests perilacunar osteolysis. These unusually wide lacunae were also noticed in bone biopsies, taken from three cosmonauts unfortunately victimized during a fatal descent in June 1973.(Yagodovsky and Gorokhova, unpublished observations in ref. 93)
The Cosmos 782 biosatellite had a total of 25 rats on board. It was the first flight with on board 1´g and 0.6´g centrifuges. Unfortunately, the centrifuge samples were not used for the bone studies as discussed below.
Tetracycline was used to determine bone mineral apposition rate.(42) Rats were injected, 3 days before launch and 3 days after reentry with this bone seeking label, which gives makes it possible to estimate the bone formation rate during the experimental period. From histomorphological observations it was concluded that bone formation was reduced during flight, however, most of the parameters returned towards control values during a 26 day post flight period. Also arrest lines, consisting of a less mineralized and biochemically inferior matrix, appeared to be more frequently present in bones from flight animals of Cosmos 782(42,74) and Cosmos 936(74) compared to controls. The abnormal alignment of the collagen fibers and the reduced crystallite content of arrest lines is expected to reduce tensile and shear strength of the bone.(74)
Cosmos 936 accommodated 30 rats in total. Twenty stayed under mg, the remaining 10 were placed on the on board 1´g centrifuges. It was shown that the mechanical quality of bone from microgravity animals had diminished after spaceflight.(69) While there were differences between the microgravity group and the in flight 1´g group as well as the synchronous group, no significant differences were found between the synchronous and the in-flight 1´g samples regarding various mechanical parameters. Most of the parameters analyzed had returned to control values at 25 days post flight.(69) Also periosteal bone formation in rat tibia was decreased in spaceflight samples compared to Earth based controls.(68)
In contrast to the two preceding missions Cosmos 1129 had no on board 1´g centrifuge. It housed a total of 37 young male rats, divided in 5 groups. After this 18.5 day flight the animals were killed at various times between 7 hours to 29 days post flight. In general lower calcium contents were found in flight samples.(17,19,29) This was confirmed during the Cosmos 1514 mission.(6) Bone histomorphological methods have been applied by Vico et al. for Cosmos 1514(76) Cosmos 1667,(77,79) Cosmos 1887(78) and Cosmos 2044.(80) From these experiments it is clear that bone formation is diminished during orbital spaceflight. Some of these light microscopical studies also revealed an increased number of osteoclasts (Cosmos 1514 and 2044). The mechanical properties as well as calcium and collagen Type-I contents were diminished after the 5 days Cosmos 1514 flight.(6,52,53) This reduction in rat femur collagen Type-I, and Type-III, content was affirmed during the Cosmos 1667 flight.(54) Since there was no change in skin collagen Type-I and III content it was argued by the authors that there was no systemic influence responsible for the reduction in bone collagen, suggesting a specific effect of spaceflight on bone.
Several papers have been published on the Cosmos 1887 flight. Cosmos 1887 contained 10 rats and 2 rhesus monkeys. The spacecraft landed in bad weather in Siberia. Because of this the recovery time was quite long for this particular flight, at least 48 hours. Various techniques have been used to study bone tissues from this flight. Mechanical tests(97), light microscopical histomorphometry, (14,16,18,22,31,78,95) and measurements of the content of various organic and inorganic compounds were performed.(41,66,97) In general the data indicates reduced mechanical, structural and / or biochemical competence of flight bones.
Out of the ten rats flown on Cosmos 2044 five were used for bone related studies. Comparable techniques as used for Cosmos 1887 were applied. After the 14 days flight similar results were reported as for previous flights. In Cosmos 1887 as well as 2044 both weightbearing and non-weightbearing bones in the rat skeleton revealed signs of osteopenia. A significant increase in calcium content of flight long bones has never been reported.
Numerous cosmonauts have already occupied the Russian space station Mir and its predecessor, Saljut-7. The nine individual cosmonauts, examined in a study by Oganov et al.(46) are only a fraction of the total number of visitors to these space stations. In this study there was, surprisingly, no clear change in vertebral bone mineral density in cosmonauts who lived under near weightlessness conditions for up to 150 and 237 days. However, reductions of bone mineral density in pelvis, femoral neck and trochanter were reported by Schneider et al. in Mir crew after a maximum of 312 days in flight.(62)
Up till now, the US has flown two SpaceLab missions, launched with the Space Shuttle, completely dedicated to microgravity life sciences, SpaceLab-3 (SL-3) and SL-2. An advantage of Shuttle missions above Cosmos flight is that Shuttle flights are manned. Crew members can give attention to the hardware and the animals, thereby promoting the well being of in flight animals. Also, the launch and reentry characteristics for a typical Shuttle mission are less harsh compared to a Cosmos lift-off. A disadvantage, especially with older orbiters, is the relatively short duration flights, up to about 10 days.
A total of 8 non-human primates and 72 rats were flown on SpaceLab-3 and SpaceLab-2 missions. About 15 rodents were used for bone related studies. There were no launch and reentry simulations for the animals, also no on board 1´g centrifuge. After the 7 days SL-3 mission a clear reduction in bone mechanical strength was reported in rat vertebrae and humeri(47,63) combined with decreased mineralization parameters at various sites in the skeleton.(15,47,65,92) Bone related hormones were screened in the astronauts.(44) During the SL-2 mission, only a transient increase in 1,25 dihydroxy vitamin D levels was found which could have induced increased bone remodeling, all other parameters remaining unchanged. Recently, some cellular parameters of bone formation, collagen Type-I and osteocalcin mRNA levels, have been studied by Backup et al.(3,4) They reported a decrease in both parameters after a 10 day Shuttle flight in ulnae periostea of the rat, while the osteocalcin mRNA level was also reduced after a 4 day mission.(4)
1.2 General Conclusion
The total number of flight individuals used for bone related studies during the first 30 years of spaceflight, included in this overview, are about 90 rats, 5 non-human primates and 26 humans (see Table-1.II). From these studies some general conclusions can be drawn:
1: During spaceflight less calcium is absorbed and / or more calcium is excreted, both resulting in net calcium loss. This was measured either in total body or at various anatomical sites during metabolic studies or various X-ray analysis. An increased mineralization in flight long bones has never been reported.(6,15,17,19,37,38,39,41,42,46,47,50,57,62,68,74,81,82,83,87,93)
2: Bone mechanical properties are diminished after spaceflight, especially in weight bearing bones.(6,47,63,69,71,97) Although most changes after spaceflight have been found in the weight bearing skeleton, also non-weight bearing bones seems to be at risk.(64,66) This could mean that systemic factors also play a role in the effects of spaceflight conditions on bone.
3: Biochemical changes of the skeletal organic matrix compounds have been reported. Changes in the content of keratosulphate,(17) reductions of Type-I and / or Type III collagen(41,52,53,54) and increases in total peptide and peptide soluble collagen content have been reported.(52,54) In addition wider collagen fibers were found in cartilage.(16) Reduced osteocalcin content,(41) and down regulated mRNA levels of osteocalcin and collagen Type-I were also reported.(4) The shifted calcium / glycosaminoglycan ratio(17) and retarded maturation of protein components(53) are indications for a retarded maturation of the bone matrix. Changes in the maturation of skeletal tissues after spaceflight was also suggested by Simmons et al.(65,66)
4: Histomorphological studies have shown a reduced trabecular bone volume(18,29,31,77,79,80,92,93,96) or reduced cortical cross sectional area,(63) both resulting in reduced mechanical properties of bone.
5: Overall longitudinal growth of long bones is affected under spaceflight conditions sometimes related to decrease in total body weight. Decreases were reported in humeri and tibial long bone lengths(15,63) as well as reductions in lengths of the different growthplate zones within the proximal tibia of the rat.(15) Reduced total body weights have been reported for humans(57,87) as well as for rats.(97)
6: There are numerous constraints involved in spaceflight research. The first problem is the restricted number of flight opportunities, aggravated by the restricted number of samples per flight. The limited number of samples is a particular dilemma in human physiology studies. To increase scientific output, animals or samples are often shared between various research groups. While being favorable with respect to the number of participants to a particular flight, tissue sharing may also imply serious restrictions in the choice of techniques. Favorable experimental procedures for one particular study may be disastrous for others, resulting in reduced scientific output and quality.
An other problem, especially relevant to Cosmos flights, is the long recovery time. This time span is important since cellular processes will continue while returned on Earth, in unit gravity. When the recovery time is long compared to the speed of the processes studied, a possible microgravity effect may have been obscured. A solution to these problems is the in flight samples preparation, as was performed recently during the SLS-2 mission (STS-58, October 1993).
Apart from the flight constraints, there is a great variability in age or total body weight of the rats used for the various spaceflight studies. With this irregularity, an additional variable is introduced. During the Cosmos flights rats between 63 and 115 days old have been used. This problem of variation in age was addressed by using rats at two different ages during the SL-3 flight. Particularly in bone, there is a distinct difference in metabolic processes between young and adult bone. Also the 'lag-time' time difference between the flight and synchronous or ground controls, sometimes more then 5 days,(42) contributes to differences and variations.
It is not clear what the long term effects of spaceflight will be on the metabolic processes of growth and remodelling within the human skeleton. Although there have been in flight training programs(24,26) and some pharmacological countermeasures have been proposed,(70,88) very little is documented in the literature about the final impact of these procedures on bone tissue. Therefore, in spite all efforts, very little is known about effective countermeasures for bone loss due to spaceflight.(48,88) It is quite likely that different procedures have to be adapted to counteract the tissue loss in muscle compared to bone tissue.
For the maintenance of skeletal integrity, as indicated in
the beginning of this paper, may be not the frequency and endurance of in flight
training cycles and prophylactics (cycle ergonometers, treadmills and 'penguin
suits') but more the level of impact (peak strain value) of the loads could
be an important factor for remaining bone integrity in space.
Although microgravity simulation studies like bedrest,(12,36) denervation,(40,86,94) and tail suspension(25,43,67,79) contribute to the knowledge of the impact of decreased mechanical loads upon the vertebrate skeleton, with the realization of the international space station Alpha at the end of this century (a combined American, European, Japanese, and Russian initiative) more data will become available to further characterize the changes seen in human and animal bone under near weightlessness conditions. Prolonged periods in space combined with the increased number of astronauts will allow to develop countermeasures for musculo-skeleton deconditioning. These countermeasures might be used in future treatment programs for terrestrial immobilization osteoporosis.
1.3 Scope of this thesis
The majority of reports concerning bone and spaceflight reveal
loss of bone mass and strength as a result of near weightlessness. It is not
clear, however, from these in vivo studies, whether these changes are
due to direct effects of microgravity on the skeleton, i.e. loss of load
bearing function, or whether they are (also) the result of changes in systemic
factors such a calcium regulating hormones(2,44) or psychological
stress factors(42,51) which are reported to be changed in rats after
flight.
With an in vitro experiment one can distinguish between the local influences of mechanical forces and changes due to systemic factors like hormones. The aim of the experiments reported in this thesis was to setup an in vitro test system using skeletal tissues to test whether skeletal metabolism under microgravity is changed due to the changed mechanical environment of space. The results of such a study may contribute to the understanding of the processes underlying immobilization osteoporosis on Earth. In the latter disease bone quantity diminishes as a result of lack of mobility due to a sedentary life related to for example old age, bed-rest and confinements to wheelchairs. At present this problem draws special attention, due to the increasing size of the elderly population within our society.
To address the relation between gravity and bone, a series of studies have been performed on the influence of a range of gravitational forces on growth and metabolism of skeletal tissue in vitro. We used whole organ cultures of fetal mouse metatarsal long bone rudiments, since it has been shown these are responsive to changes in their mechanical environment.(5,9,33,34) To allow microgravity studies we adapted the normal laboratory culture procedures for experiments in the US Space Shuttle (STS-42, IML-1 January 22 1992) and in the Russian Biocosmos satellite (Bion-10, December 29 1992). In concert with these microgravity studies hypergravity experiments were also performed.
Charter 2 describes the outset of a spaceflight experiment. It addresses the so typical interaction of biological and engineering aspects needed for microgravity experiments. This second chapter reports the preparation of an experiment to be performed in the ESA Biorack facility of Spacelab aboard the Space Shuttle, the results of which are found in Chapter 3. A confirmation of the results from the Space Shuttle experiment is described in Chapter 5, in which the same type of fetal long
Table 1.II. Characteristics of the various flights.
|
Flight number: |
Species: |
Number of samples: |
Sex: |
Age: at launch = L
Age: at death = †
(days): |
Weight at launch = L
Weight at death = †
(grams): |
|
Gemini-4 |
human |
Flight:2 |
male |
adult |
|
|
Gemini-5 |
human |
Flight: 2 |
male |
adult |
|
|
Gemini-6 |
human |
Flight: 2 |
male |
adult |
|
|
Gemini-7 |
human |
Flight: 2 |
male |
adult |
|
|
Biosatellite III |
monkey |
Flight: 1 |
male |
|
6 kg |
|
Apollo 17 |
human |
Flight: 3 |
male |
adult |
|
|
SkyLab-II |
human |
Flight: 3 |
male |
adult |
|
|
SkyLab-III |
human |
Flight: 3 |
male |
adult |
|
|
SkyLab-IV |
human |
Flight: 3 |
male |
adutl |
|
|
Cosmos 605 |
rat |
Flight: 5 |
? |
? |
? |
|
Cosmos 782 |
rat |
Basal: 6 (B)
Flight: 6 (F)
Synchronous:6 (S)
Vivarium: 6 (V) |
male |
Flight: 63 (L)
Synchronous: 63 (L)
Vivarium: 63 (L) |
all 215 (L) |
|
Cosmos 936 |
rat |
Flight: 10
Flight 1xg: 10
Synchronous: 10
Vivarium: 10 |
male |
63 (L)
|
202±13.9 (L) |
|
Cosmos 1129 |
rat |
Flight: 5
Synchronous: 7
Vivarium: 4 |
male |
Flight: 133 (†)
Synchronous: 138 (†)
Vivarium: 135 (†) |
290 (L)
F: 349±4 (†)
S: 359±2 (†)
V: 349±4 (†) |
|
Cosmos 1514 |
rat |
Basal: ?
Flight: 5-10
Synchronous:5-10
Vivarium: 5 |
female |
83 (L) |
295 (L)
F: 300 (†)
S: 360 (†) |
|
Cosmos 1667 |
rat |
Basal: ?
Flight: 7-10
Synchronous:7-10
Vivarium: 7 |
male |
105 (L) |
Flight: 304±46 (†)
Synchronous: 334±16 (†) |
|
Cosmos 1887 |
rat
monkey |
Basal: 5
Flight: 5
Synchronous: 5
Vivarium: ?
monkey: 2 |
male
male |
Basal: 85 (†)
Flight: 105 (†)
Synchronous: 111 (†)
Vivarium: 108 (†) |
B: 316±8 (†) :L-5 d
F: 303±2 (†) :R+2.4 d
S: 349±8 (†) :R+5 d
V: 342±8 (†) R+3-4 d
monkey: 4 kg |
|
Cosmos 2044 |
rat
monkey |
rat:
Flight: 5
Synchronous: 5
Vivarium: 5
monkey: 2 |
male
male |
rat: B: 108 (†)
F: 123 (†)
S: 126 (†)
V: 129 (†)
monkey: 3.5 years |
rat: B: 320±4 (†)
F: 338±2 (†)
S: 343±7 (†)
V: 363±2 (†)
monkey: 3.8-3.9 kg |
|
Salyut-7 / Mir |
human |
Flight: 9 |
? |
adult |
|
|
SpaceLab-3 (SL-3) |
rat |
Flight 1: 5
Flight 2: 6
Flight Simul 1: 5
Flight Simul 2: 6 |
male |
Flight 1: 84 (†)
Flight 2: 56 (†)
Fl. Sim. 1: 84 (†)
Fl. Sim. 2: 58 (†) |
Basal: 200±6
Flight 1: 384±9 (†)
Flight 2: 194±10 (†)
Fl. Sim.1: 395±23 (†)
Fl. Sim.2: 198±5 (†) |
|
SpaceLab-2 (SL-2) |
human |
Flight: 4 |
|
|
|
|
STS-41 |
rat |
Flight: 8
Flight Control: 12 |
male |
Flight: 8
Fl. Control: 12 |
Flight: 151±4 (†)
Fl. Control: 164±2 (†) |
|
STS-52 |
rat |
Flight: 6
Flight Control: 6 |
male |
Flight: 6
Fl. Control: 6 |
Flight: 260±3 (†)
Fl. Control: 265±2(†) |
bones have been cultured on board a Russian Biocosmos (Bion-10) satellite. For the latter experiments a series of biocompatibility tests were performed on an automated tissue culture device to be used for this Bion-10 flight. Chapter 4 reveals the use of polysulphone as the prime material for constructing this tissue culture device.
Complementary to the microgravity studies, hypergravity experiments
are described in Chapter 6. In this study fetal mouse long bones were subjected
to extra g-forces of 2.2 to 3.1´g. In Chapter 7 provides a summary and general
discussion on all the work presented in this thesis. Appendix A describes a
new research tool for gravitational research. In Appendix B some physical phenomena
involved in microgravity research in relation to in vitro cell cultures
are addressed. Finally, Appendix C is a detailed summary of literature concerning
spaceflight and bone. It can be used alongside the literature overview in the
first chapter.
1.4 REFERENCES
1 Aloia J.F., Cohn S.H., Babu Th., Abesamis C., Kalici N., Ellis K. Skeletal
mass and body composition in marathon runners. Metablism 27, 1793-1796, 1978.
2 Arnaud S.B., Fung P., Popova I.A., Morey-Holton E.R., Grindeland R.E. Circulating
parathyroid hormone and calcitonin in rats after spaceflight. J. Appl. Physiol.
73, 169S-173S, 1992.
3 Backup P., Wakley G.K., Harris S., Spelsberg T.C., Turner R.T. Spaceflight
results in decreased gene expression for osteoclacin in bone and actine in muscle.
J. Bone Min. Res. S122 (no. 119), 1992.
4 Backup P., Westerlind K., Harris S., Spelberg T., Kline B., Turner R. Spaceflight
results in reduced mRNA levels for tissue-specific proteins in musculoskeletal
system. Am. J. Physiol. 226, E567-E573, 1994.
5 Bagi C., Burger E.H. Mechanical Stimulation by Intermittent Compression Stimulates
sulfate Incorporation and Matrix Mineralization in Fetal Mouse Long-bone Rudiments
Under Serum-Free Conditions. Calcif. Tissue Int. 45, 342-347, 1989.
6 Bakulin A.V., Ilyan E.A., Organov V.S., Lebedev V.I. The state of bones of
pregnant rats during an acute stage of adaptation to weightlessness. ESA SP-237,
Proc. 2nd Intern. Conf. Space Physiol. (Toulouse, France, 20-22 Nov. 1985),
225-229, 1985.
7 Ballard R.W., Connolly J.P. U.S./U.S.S.R. joint research in space biology
and medicine on Cosmos satellites. FASEB J. 4, 5-9, 1990a.
8 Ballard R.W., Mains R.C. Animals experiments in space; a brief overview. Fundamentals
of space biology. eds. Asashima M. and Malacinski G.M. Japan Sci. Press, Tokyo/Springer-Verlag,
Berlin, 21-41, 1990b.
9 Burger E.H., Veldhuijzen J.P., Klein-Nulend J., Van Loon J.J.W.A. Osteoclastic
Invation and Mineral Resorption of Fetal Mouse Long Bone Rudiments are inhibited
by culture under intermittent compressive force. Connective Tissue Research
20, 131-141, 1989.
10 Cann Ch.E., Adachi R.R. Bone resorption and mineral excretion in rats during
spaceflight. Am. J. Physiol. 22, R327-R331, 1983.
11 Dalsky G.P., Stocke K.S., Ehsani A.A., Lee W.C., Birge S.J. Weightbearing
exercise training and lumbar bone mineral content in postmenopausal women. Ann.
Intern. Med. 108, 824-828, 1988.
12 Donaldson Ch.L., Hulley S.B., Vogel J.M., Hattner R.S., Bayers J.H., McMillan
D.E. Effect of prolonged bed rest on bone mineral. Metabolism 19, 1071-1084,
1970.
13 Doty S.B. Morphologic and Histochemical studies of bone cells from SL-3 rats.
Physiologist 28, S-225-S226, 1985.
14 Doty S.B., Morey-Holton E.R., Durnova G.N., Kaplansky A.S. Morphological
studies of bone and tendon. J. Appl. Physiol. 73, 10S- 13S, 1992.
15 Duke J., Janer L., Campbell M., Morrow J. Microprobe analyses of epiphyseal
plates from spacelab 3 rats. Physiologist 28(6), S-217-218, 1985.
16 Duke P.J., Durnova G., Montufar-Solis D. Histomorphometric and electron microscopic
analyses of tibial epiphyseal plates from Cosmos 1887 rats. FASEB J. 4, 41-46,
1990.
17 Eurell J.A., Kazarian L.E. Quantitative histochemistry of rat lumbar vertebrae
following spaceflight. Am. J. Physiol. 244, R315- R318, 1983.
18 Földes I., Rapcsák M., Szilágyi T., Organov V.S. Effects
of space flight on bone formation and resorption. Acta Physiologica Hungarica
75, 271-85, 1990.
19 France E.P., Oloff C.M., Kazarian Bone mineral analysis of rat vertebra following
spaceflight COSMOS 1129. Physiologist 25, S147-S148, 1982.
20 Frost H.M. Vital biomechanics : proposed general consepts for skeletal adaptations
to mechanical usage. Calcif. Tissue Int. 42, 145- 156, 1988.
21 Galilei Galileo. Two new sciences. In: "The second day", translated by Stillman
Drake. The University of Wisconsin Press. page 109-146, 1974.
22 Garetto L.P., Gonsalves M.R., Morey E.R., Durnova G., Roberts W.E. Preosteoblast
production 55 hours after a 12.5-day spaceflight on Cosmos 1887. FASEB J. 4,
24-38, 1990.
23 Garetto L.P., Morey E.R., Durnova G.N., Kaplansky A.S., Roberts W.E. Preosteoblast
production ins COMSOS 2044 rats: short-term recovery of osteogenic potential.
J. Apl. Physiol. 73, 14S-18S, 1992.
24 Garshnek V. Soviet space flight: the human element. ASGSB Bulletin, 67-80,
May 1988.
25 Globus R.K., Bikle D., Halloran B., Morey-Holton E. Skeletal response to
dietary calcium in a rat model simulating weightlessness. J. Bone Min. Res.
1, 191-197, 1986.
26 Greenleaf J.E., Bulbulian R., Bernauer E.M., Haskell W.L., Moore T. Exercise-training
protocols for astronauts in microgravity. J. Appl. Physiol. 67, 2191-2204, 1989.
27 Grindeland R.E., Popova I.A., Vasques M., Arnaud S.B. Cosmos 1887 mission
overview: effects of microgravity on rat body and adrenal weights and plasma
constituents. FASEB J. 4, 105-109, 1990.
28 Grindeland R.E., Ballard R.W., Conolly J.P., Vasques M.F. COSMOS 2044 mission,
Overview. J. Appl. Physiol. 73, 1S-3S, 1992.
29 Jee W.S.S., Wronski T.J., Morey E.R., Kimmel D.B. Effects of spaceflight
on trabecular bone in rats. Am. J. Physiol. 244, R310- R314, 1983.
30 Johnson P.C., Leach C.S., Rambaut P.C. Estimates of fluid and energy balances
of Apollo 17. Aerospace Med. 44, 1227-1230, 1973.
31 Kaplansky A.S., Durnova G.N., Ilyina-Kakueva E.I. Histomorphometric analysis
of bones of Cosmos-1887 rats. Physiologist 33, s20-s22, 1990.
32 Kaplansky A.S., Durnova G.N., Burkovskaya T.E., Vorotnikova E.V. The effect
of microgravity on bone fracture healing in rats flown on Cosmos 2044. Physiologist
34, S196-S199, 1991.
33 Klein Nulend J., Veldhuijzen J.P., Burger E.H. Increased calcification of
growth plate cartilage as a result of compressive force in vitro. Arthritis
Rheum. 29, 1-9, 1986.
34 Klein Nulend J., Veldhuijzen J.P., Strien M.E. van, Jong M. de, Burger E.H.
Inhibition of osteoclast bone resorption by mechanical stimulation in vitro.
Arthritis Rheum. 3366-72, 1990.
35 Lanyon L.E. Functional strain as a determinant for bone remodeling. Calcif.
Tissue Int. 36, S56-S61, 1984.
36 LeBlanc A.D., Schneider V.S., Evans H.J., Engelbretson D.A., Krebs J.M. Bone
mineral loss and recovery after 17 weeks of bed rest. J. Bone Min. Res. 5, 843-850,
1990.
37 Lutwak L., Whedon G.D., Lachance P.A., Reid J.M., Lipscomb H.S. J. Clin.
Endocr. 29, 1140-1156, 1969.
38 Mack P.B., LaChance P.A., Vose G.P., Vogt F.B. Bone demineralization of foot
and hand of gemini-titan IV, V and VII astronauts during orbital flight. Am.
J. Roentgenol., Rad. Therapy & Nuclear Med. 100, 503-511, 1967.
39 Mack P.B. Bone density changes in a Macaca Nemestrina Monkey during the Biosatellite
III project. Aerospace Med. 42, 828- 833, 1971.
40 Marotti G., Delrio N., Marotti F., Fadda M. Quantitative analysis of the
bone destroying activity of osteocytes and osteoclasts in experimental disuse
osteoporosis. Ital. J. Orthop. Traumatol. 5, 225-240, 1979.
41 Mechanic G.L., Arnaud S.B., Boyde A., Bromage T.G., Buckendahl P., Elliott
J.C.,Katz E.P.,Durnova G.N. Regional distribution of mineral and matrix in the
femurs of rats flown on Cosmos 1887 biosatellite. FASEB J. 4, 34-40, 1990.
42 Morey E.R., Baylink D.J. Inhibition of bone formation during space flight.
Science 201, 1138-1141, 1978.
43 Morey E.R. Spaceflight and bone turnover: Correlation with a new rat model
of weightlessness. BioScience 29, 168-172, 1979.
44 Morey-Holton E.R., Schnoes H.K., DeLuca H.F., Phelps M.E., Klein R.F., Nissenson
R.H., Arnaud C.D. Vitamin D metabolites and bioactive parathyroid hormone levels
during Spacelab 2. Aviat. Space Environ. Med. 59, 1038-1041, 1988.
45 Nicogossian A.E. Overall physiological response to space flight. In: Space
Physiology and Medicine, Lea & Febiger, page 148, 1989.
46 Oganov V.S., Rakhmanov A.S., Novikov V.E., Zatsepin S.T., Rodionova S.S.,
Cann Ch. The state of human bone tissue during space flight. Acta Astronautica
23, 129-133, 1991.
47 Patterson-Buckendahl P.E., Arnaud S.B., Mechanic G.L., Martin R.B, Grindeland
R.E., Cann C.E. Fragility and composition of growing rat bone after one week
in spaceflight. Am. J. Physiol. 252, R240-R246, 1987.
48 Pavy-Le Traon A., Güell A., Saivin S., Houin G., Soulez-Larivière
C., Pujos M. The use of medicaments in space. Therapeutic measures and potential
impact of pharmacokinetics due to weightlessness. ESA Journal, 18, 33-50, 1994.
49 Perdrini-Mille A., Maynard J.A., Durnova G.N., Kaplansky A.S., Pedrini V.A.,
Chung C.B., Fedler-Troester J. Effects of microgravity on the composition of
the intervertebral disk. J. Appl. Physiol. 73, 26S-32S, 1992.
50 Pitts G.C., Ushakov A.S., Pace N., Smith A.H., Rahlmann D.F., Smirnova T.A.
Effects of weightlessness on body composition in the rat. Am. J. Physiol. 244,
R332-R337, 1983.
51 Popova I.A., Afonin B.V., Davydova N.A., Grigoriev A.I. Hormonal regulation
in spaceflights of varying duration. Physiologist 30, S42-S44, 1987.
52 Pospisilova J., Serova L.V., Pospisil M. The effects of spaceflight on the
distribution of collagen types in bones of pregnant rats. Scr. Med. (Brno) 59,
277-282, 1986.
53 Pospisilova J., Pospisil M., Serova L.V. Deviation in the developmental dynamics
of the protein composition of the connective tissue of rats gestated during
the flight in the biological satellite Kosmos 1524. Scr. Med. (Brno) 60, 277-288,
1987.
54 Pospisilova J., Pospisil M., Serova L.V. Effect of spaceflight on collagen
pepsin solubility and collagen type distribution in femoral bone and skin of
rats. Physiologist 31, S32-S33, 1988.
55 Pruitt L.S., Jackson R.D., Bartels R.L., Lehnhard H.J. Weight- training effects
on bone mineral density in early postmemopausal women. J. Bone Min. Res. 7,
179-185, 1992.
56 Raab D.M., Crenchaw T.D., Kimmel D.B., Smith E.L. A histomorphometric study
of the cortical bone activity during increased weight-bearing exercise. J Bone
Min Res 6, 741-749, 1991.
57 Rambaut P.C., Leach C.S., Johnson P.C. Calcium and phosphorus change of the
Apollo 17 crew members. Nutr. Metabol. 18, 62-69, 1975.
58 Roux W. Gesammelte Abhandlung über Entwicklungsmechanic der Organismen.
Vol I and II. Engelmann W., Leipzig, 1895.
59 Rubin C.T., Lanyon L.E. Regulation of bone mass by peak strain magnitude.
Trans. Orthop. Res. Soc., 1983.
60 Rubin C.T., Lanyon L.E. Regulation of bone formation by applied dynamic loads.
J Bone Joint Surg 66, 397-402, 1984a.
61 Rubin C.T., Skeletal strain and the functional significance of bone architecture.
Calcif Tissue Int 36, S11-S18, 1984b.
62 Schneider V., Oganov V., LeBlanc A., Rakhmanov A., Bakulin A., Grigoriev
A., Varonin L. Space flight bone loss and change in fat and lean body mass.
J. Bone Min. Res. abstract 117, S122, 1992.
63 Shaw S.R., Vailas A.C., Grindeland R.E., Zernicke R.F. Effects of a 1-wk
spaceflight on morphological and mechanical properties of growing bone. Am.
J. Physiol. 254, R78-R83, 1988.
64 Simmons D.J., Russell J.E., Winter F., Tran Van P., Vignery A., Baron R.,
Rosenberg G.D., Walker W.V. Effect of spaceflight on the non- weight-bearing
bones of the rat skeleton. Am. J. Physiol. 244, R319- R326, 1983.
65 Simmons D.J., Russell J.E., Grynpas M.D. Bone maturation and quality of bone
material in rats flown on the space shuttle 'Spacelab 3 mission'. Bone Min.
1, 485-493, 1986.
66 Simmons D.J., Grynpas M.D., Rosenberg G.D. Maturation of bone and dentin
matrices in rats flown on the Soviet biosatellite Cosmos 1887. FASEB J. 4, 29-33,
1990.
67 Simske S.J., Guerra K.M., Greenberg A.R., Luttges M.W. The physical and mechanical
effects of suspension-induced osteopenia on mouse long bones. J. Biomechanics
25, 489-499, 1992.
68 Spector M., Turner R.T., Morey-Holton E., Baylink D.J., Bell N.H. Arrested
bone formation during spaceflight results in a hypomineralized skeletal defect.
Physiologist 26, S110-S111, 1983.
69 Spengler D.M., Morey E.R., Carter D.R., Turner R.T., Baylink D.J. Effects
of spaceflight on structural and material strength og growing bone. P.S.E.B.M.
174, 224-228, 1983.
70 Stepaniak P.C., Furst J.J., Woodard D. Anabolic steroids as a countermeasure
against bone demineralization during space flight. Aviat. Space Environ. Med.
0, 1986.
71 Stupakov G.P., Kazeikin V.S., Morukov B.V. Microgravity-induced changes in
human bone strenght. Physiologist 32, S41-S44, 1989.
72 Taylor G.R. Overview of spaceflight immunology studies. J. Leukocyte Biol.
54, 179-188, 1993.
73 Tilton F.E., Degioanni J.J.C., Schneider V.S. Long-term follow-up of skylab
bone demineralization. Aviat. Space Environ. Med. 11, 1209-1213, 1980.
74 Turner R.T., Bell N.H., Duvall P., Bobyn J.D., Spector M., Morey- Holton
E., Baylink D.J. Spaceflight results in formation of defective bone (42215).
Proc. Soc. Exp. Biol. Med. 180, 544-549, 1985.
75 Vailas A.C., Vanderby R., Martinez D.A., Ashman R.B., Ulm M.J., Grindeland
R.E., Durnova G.N., Kaplansky A.S. Adaptations of young adult rat cortical bone
to 14 days of spaceflight. J. Appl. Physiol. 73, 4S-9S, 1992.
76 Vico L., Chappard D., Alexandre C., Palle S., Minaire P., Riffat G., Novikov
V.E., Bakulin A.V. Effects of weightlessness on bone mass and osteoclast number
in pregnant rats after a five-day spaceflight (Cosmos 1514). Bone 8, 95-103,
1987.
77 Vico L., Chappard D., Palle S., Bakulin A.V., Alexandre C. Trabecular bone
remodeling after seven days of weightlessness exposure (biocosmos 1667). Am.
J. Physiol. 255, r243-r247, 1988.
78 Vico L., Bourrin J.M., Very D., Chappard D., Alexandre C. Bone adaptation
to real and simulated microgravity. Proc. 4th. Europ. Symp. Life
Sci. Res. Space. Triest, Italy, ESA-SP-307, 359-361, May 1990.
79 Vico L., Novikov V.E., Very J.M., Alexandre C. Bone hisomorphometric comparison
of rat tibial metaphysis after 7 day tail suspension vs. 7 day spaceflight.
Aviat. Space Environ. Med. 62, 26-31, 1991.
80 Vico L., Bourrin S., Genty C., Palle S., Alexandre C. Histomorphometric analyses
of cancellous bone from Cosmos 2044 rats. J. Appl. Physiol. 75, 2203-2208, 1993.
81 Vogel J.M., Whittle M.W. Bone mineral changes: The second manned Skylab mission.
Aviat. Space Environ. Med. 47(4), 396-400, 1976a.
82 Vogel J.M., Whittle M.W. Bone mineral content changes in the Skylab astronauts.
Am. J. Roentgenol., Rad. Therapy & Nuclear Med. 126, 1296-1297, 1976b.
83 Vose G.P. Review of roentgenographic bone demineralization studies of the
gemini space flights. Am. J. Roentgenol., Rad. Therapy & Nuclear Med. 121,
1-4, 1974.
84 Ward F.O. Outlines of human osteology. 3rd edition, Henry Renshaw,
London, 1876.
85 Weingart H., Kleber M., Geissler H., Wachtel E., Hecht K., Denissova L.,
Sergejev I., Serova L. The influence of a one-week space flight on teeth and
jaw bones of wistar-rats - Cosmos 1514 and Cosmos 1667. Physiologist 31, S34-S35,
1988.
86 Weinreb M., Rodan G.A., Thompson D.D. Osteopenia in the immobilized rat hind
limb is associated with increased bone resorption and decreased bone formation.
Bone 10, 187-194, 1989.
87 Whedon G.D., Lutwak L.,Reid J., Rambaut P., Whittle M., Smith M., Leach C.
Mineral and nitrogen balance study, results of metabolic observations on Skylab
II 28-day orbital mission. Acta Astronautica 2, 297-309, 1975.
88 Whedon G.D., Heaney R.P. Effects of physical inactivity, paralysis and weightlessness
on bone growth. In: Bone Vol. 7(3) edt. Hall. CRC Press, Inc. 57-77, 1993.
89 Wolff J.D. Das Gezetz der Transformation der Knochen, Berlin, A. Hirschwald,
1892.
90 Wronski T.J., Morey E.R. Alterations in calcium homeostasis and bone during
actual and simulated space flight. Med. Sci. Sports Exerc. 15, 410-414, 1983a.
91 Wronski T.J., Morey E.R. Effects of spaceflight on periosteal bone formation
in rats. Am. J. Physiol. 244, R305-R309, 1983b.
92 Wronski T.J., Morey-Holton E.R., Doty S.B., Maese A.C., Walsh C.C. Histomorphometric
analysis of rat skeleton following spaceflight. Am. J. Physiol. 252, R252-R255,
1987.
93 Yagodovsky V.S., Triftanidi L.A., Gorokhova G.P. Space flight effects on
skeletal bones of rats (Light and electron microscopic examiniation). Aviat.
Space Environ. Med. 47, 734-738, 1976.
94 Yeh J.K., Liu C.C., Aloia J.F. Effects of exercise and immobilization on
bone formation and resorption in young rats. Am. J. Physiol. 264, E182-E189,
1993.
95 Zerath E., Nogues C., Borne M., Soudraine P. Bone effects of 13 days of weightlessness
on rat and monkey: some results of Biocosmos 1887 and ground simulation. Physiologist
33, S94-S95, 1990.
96 Zerath E., Holy X., Malouvier A., Caissard J-C., Nogues C. Rat and monkey
bone study in the biocosmos 2044 space experiment. Physiologist 34, S194-S195,
1991.
97 Zernicke R.F., Vailas A.C., Grindeland R.E., Kaplansky A.S., Salem G.J.,
Martinez D.A. Spaceflight effects on biomechanical and biochemical properties
of rat vertebrae. Am. J. Physiol. 258, R1327- R1332, 1990.
The following papers provide more general data concerning the various flights
discussed and used for this overview but which are not (always) referred to
individually:
98 Ballard R.W., Connolly J.P. U.S./U.S.S.R. joint research in space biology
and medicine on Cosmos satellites. FASEB J. 4, 5-9, 1990a.
99 Ballard R.W., Mains R.C. Animals experiments in space; a brief overview.
Fundamentals of space biology. eds. Asashima M. and Malacinski G.M. Japan Sci.
Press, Tokyo/Springer-Verlag, Berlin, 21-41, 1990b.
100 Grindeland R.E., Ballard R.W., Conolly J.P., Vasques M.F. COSMOS 2044 mission,
Overview. J. Appl. Physiol. 73, 1S-3S, 1992.
101 Grindeland R.E., Popova I.A., Vasques M., Arnaud S.B. Cosmos 1887 mission
overview: effects of microgravity on rat body and adrenal weights and plasma
constituents. FASEB J. 4, 105-109, 1990.
102 Taylor G.R. Overview of spaceflight immunology studies. J. Leukocyte Biol.
54, 179-188, 1993.
With
minor changes published
in: Jack J.W.A. van Loon, J. Paul Veldhuijzen, Elizabeth H. Burger. Bone and space
flight: an overview. in Biological and Medical Research in Space, Edt. D. Moore,
P. Bie and H. Oser. Springer-Verlag Berlin Heidelberg, Chapter 5, 259-299, 1996.

