
|
FLOW
|

|
Abstract: Bone cells, in particular osteocytes, are extremely sensitive to mechanical stress, a quality that is probably linked to the process of mechanical adaptation (Wolff's Law). The in vivo operating cell stress derived from bone loading is likely flow of interstitial fluid along the surface of osteocytes and lining cells. The response of bone cells in culture to fluid flow includes prostaglandin synthesis and expression of inducible prostaglandin G/H synthase (PGHS-2 or inducible cyclooxygenase, COX-2), an enzyme that mediates the induction of bone formation by mechanical loading in vivo. Disruption of the actin-cytoskeleton abolishes the response to stress, suggesting that the cytoskeleton is involved in cellular mechanotransduction. Microgravity has catabolic effects on the skeleton of astronauts, as well as on mineral metabolism in bone organ cultures. This might be explained simply as resulting from an exceptional form of disuse under weightlesness conditions. However, under microgravity conditions the assembly of cytoskeletal elements may be altered, as gravity has been shown to determine the pattern of microtubular orientation assembled in vitro. Therefore we hypothesize that the mechanosensitivity of bone cells is altered under microgravity conditions, and that this abnormal mechanosensation contributes to the disturbed bone metabolism observed in astronauts. In vitro experiments on the International Space Station should test this hypothesis experimentally.
Address corresponding author: Dr. J. Klein-Nulend ACTA-Vrije Universiteit Dept. of Oral Cell Biology Van der Boechorststraat 7 1081 BT Amsterdam The Netherlands Phone: +31-20-444 8660 Fax: +31-20-4448683 E-mail: J.KleinNulend@VUMC.nl
Co-Investigators: dr.ing.J.J.W.A.
van Loon, dr. J.P. Veldhuijzen, PhD student drs. R.G. Bacabac.
 Goal
Goal
In the present DELTA experiment, we wish to test the hypothesis of changed
cell mechanosensitivity under near-weightlessness conditions in a cell culture
system but with a simplified flow setup. The specific aim of this research proposal
is to test whether near-weightlessness decreases the sensitivity of chicken
osteocytes for mechanical stress through a decrease in early signaling molecules
that are involved in the mechanical loading-induced osteogenic response. Osteocytes,
the bone mechanosensitive cells par excellence, will be compared with osteoblasts
and periosteal fibroblasts. Osteocytes, osteoblasts, and periosteal fibroblasts
are cultured with or without gravity. Gravity will be applied using an onboard
centrifuge. Cell culture conditions and cell responses will be measured on-line
using nitric oxide sensors. At the end of the experiment conditioned medium
will be tested for prostaglandin and nitric oxide production. Semi-quantitative
polymerase chain reactions will be performed to study COX and NOS mRNA expression.
This taxi flight study could use existing cell culture modules and will provide
further insight in the mechanism of mechanotransduction in bone.
Introduction
It has been well documented that bone tissue is
sensitive to its mechanical environment. Subnormal mechanical stress as a result
of bedrest or immobilization results in decreased bone mass and disuse osteoporosis
(Houde et al. 1995). Spaceflight produces a unique condition of skeletal unloading
as a result of the near absence of gravity. Studies of animals and humans subjected
to spaceflight agree that near weightlessness negatively affects the mass and
mechanical properties of bone (for a review, see Van Loon et al. 1996). Although
the exact mechanism whereby bone loss as a result of spaceflight occurs is still
unknown, recent in vivo studies suggest that bone cells are directly sensitive
to near weightlessness. Using organ cultures of living bone rudiments from embryonic
mice, Van Loon et al. (1995) showed that 4 days of spaceflight inhibited matrix
mineralization, while stimulating osteoclastic resorption of mineralized matrix.
Monolayer cultures of the human osteoblastic cell line MG-63 responded to 9
days of near weightlessness with reduced expression of osteocalcin, alkaline
phosphatase, and collagen Ia1 mRNA (Carmeliet et al. 1996). Reduced prostaglandin
production was found in cultures of MC3T3-E1 osteoblastic cells exposed to 4
days of near weightlessness, probably due to inhibition of serum-induced growth
activation (Hughes-Fulford and Lewis 1996). In addition near weightlessness
induced prostaglandin E2 (PGE2) and interleukin-6 production in rat bone marrow
stroma cultures, an observation that may be related to alterations in bone resorption
(Kumei et al. 1996). These results suggest that mineral metabolism and bone
cell differentiation are modulated by near weightlessness, and that bone cells
are directly responsive to micro-g conditions. Direct responses of bone cells
to mechanical stimuli have been studied using several methods to apply mechanical
stress in vivo (for a review, see Burger and Veldhuijzen 1993). Stretching or
bending of the cell substratum has been widely used, but recent evidence indicates
that fluid flow over the cell surface may better simulate the cellular effect
of mechanical loading of bone in vivo (Cowin et al. 1991; Klein-Nulend et al.
1995; Reich et al. 1990; Turner et al. 1994; Weinbaum et al. 1994). Strain (deformation)
of the bone matrix as a result of mechanical stress in vivo causes flow of interstitial
fluid through the network of osteocyte lacunae and canaliculi (Kufahl and Saha
1990; Piekarski and Munro 1977). Weinbaum et al. (1994) used Biot's porous media
theory to relate loads applied to a whole bone to the flow of canalicular interstitial
fluid. Their calculations predict fluid shear stresses of 0.8 to 3 Pa as a result
of peak physiological loading regimes. Based on this hypothesis, we have recently
tested whether osteocytes are sensitive to fluid shear stress in vivo, and which
paracrine factors are produced in response to fluid flow. In the following we
will briefly review these studies
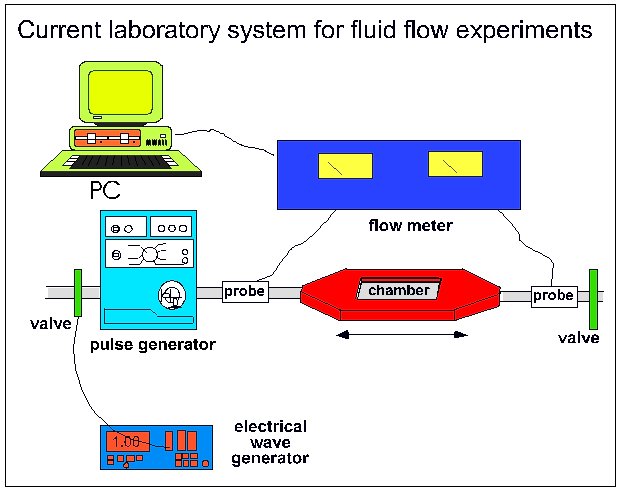 |
Figure 1:
Current fluid flow system as is it used for ongoing ground based research
in our laboratory. The main parts are the culture chamber, a pulse generator,
flow meter and a data logger (PC)
|
Pulsatile Fluid Flow
For studies on cell mechanosensitivity, a pulsatile fluid shear stress was applied
to monolayers of bone cells using the apparatus schematically shown in Figure
1. Essentially, a shear stress was applied by pumping culture medium through
a flow chamber containing a monolayer of cultured cells. The flow chamber consisted
of a machine-milled polycarbonate plate, a rectangular Dural (AlMgSi; 51ST)
gasket, and a polylysine-coated (50 mg/ml; poly-l-lysine hydrobromide, MW 15-30x104;
Sigma, St. Louis, IL) glass slide containing the cell monolayer. Polycarbonate
plate, gasket, and glass slide were assembled such that a channel was created
above the cells that was 2-4 cm wide and 0.03 cm deep. The area of cells exposed
to shear was 14 cm2. The polycarbonate plate had two manifolds through which
medium entered and left the channel. The entry port was larger than the exit
port and served as a bubble trap. During an experiment, all components were
placed in a 37°C incubator, and the medium reservoir was connected to a gassing
system that maintained a humidified atmosphere of 5% CO2 in air. Pulsatile fluid
flow (PFF) resulted from pumping the culture medium over the cells in a pulsatile
(5 Hz) manner using a revolving pump. The flow rate was monitored using a flow
probe (Figure 1). The wall shear stress on the cell monolayer was calculated
using the momentum balance for a Newtonian fluid and assuming parallel-plate
geometry. In all studies discussed here we subjected the cell monolayers to
the same magnitude of shear stress, which was calculated to be 7.2 dynes/cm2
(0.7 Pa). The flow profile was measured in the fluid circuit after the flow
chamber using an animal research flow meter (Transonic Systems Inc., Ithaca,
NJ). We observed a sinusoidal flow profile with a minimum and maximum shear
stress of respectively 2.1 and 9.9 dynes/cm2 (0.2 and 1.0 Pa), and an estimated
peak stress rate of 12.2 Pa/sec (Sterck 1996).
Response of monolayer bone
cell cultures to PFF
The theory of fluid flow-dependent mechanosensing in bone tissue assumes that
osteocytes, bone lining cells, and osteoblasts, but not osteoblast precursors
or osteoclasts, are the "professional" mechanosensor cells of bone. This is
because the flow of interstitial fluid resulting from load-induced strain is
only important in the lacunar-canalicular network, and is negligible in the
Haversian and Volkmann channels. These latter channels are much wider (about
30,000 times wider than canaliculi) and the fluid pressure in them is more uniform
as it must be almost the same as the blood pressure. To test this theory, the
mechanosensitivity of osteocytes was compared with that of osteoblasts and periosteal
fibroblasts (Klein-Nulend et al. 1995a). Cells were isolated from chicken embryo
calvariae and separated in three fractions. One fraction consisted for more
than 95% of osteocytes as a result of immunoseparation based on the osteocyte
specific antibody OB 7.3. A second fraction consisted for more than 90% of osteoblasts,
and the third fraction contained periosteal fibroblasts (Van der Plas and Nijweide
1992). The three cell types were submitted to PFF as well as to intermittent
(0.3 Hz) hydrostatic compression of 13 kPa (Klein-Nulend et al. 1986). Osteocytes,
but not osteoblasts or periosteal fibroblasts, reacted to 1 h PFF with a sustained
release of prostaglandin E2 (PGE2) (Klein-Nulend et al. 1995a). Intermittent
hydrostatic compression stimulated prostaglandin production to a lesser extent,
i.e. after 6 and 24h continuous treatment in osteocytes, and after 6 h in osteoblasts.
These data provided evidence that osteocytes, at least in chickens, are the
most mechanosensitive cells in bone, and that a fluid flow of 0.7 Pa was more
effective than hydrostatic compression of 13000 Pa. The results therefore supported
the hypothesis that strain-derived fluid flow in the lacunar-canalicular system
provides the stimulus for an adaptive response in bone.
In another study (Klein-Nulend et al. 1995b) it was shown that chicken osteocytes
but not periosteal fibroblasts responded to PFF with a rapid and transient 2
to 3-fold upregulation of nitric oxide (NO) release. The effect was transient,
reaching a maximum after 5 minutes and leveling off thereafter. A similar effect
was observed in the late-released fraction of mouse calvarial bone cells obtained
by sequential digestion (Klein-Nulend et al. 1995b). PFF also acutely stimulated
PGE2 release by mouse (Klein-Nulend et al. 1995b) and chicken (Ajubi et al.
1996) bone cells. This effect was significant after 5 to 10 minutes and continued
throughout 60 minutes of PFF treatment. Importantly, inhibition of NO release
by the competitive NO synthase inhibitor Na-monomethyl-L-arginine, prevented
the effect of PFF on NO release as well as on PGE2 release (Klein-Nulend et
al. 1995b). These results suggested that NO is another mediator of mechanical
effects on bone, and that NO release is critical for the PFF-mediated PGE2 release.
We have also shown that the rapid production of NO in human bone cells in response
to fluid flow results from activation of endothelial cells nitric oxide synthase
(ecNOS) (Klein-Nulend et al. In press).These results suggest that the response
of bone cells to mechanical stress resembles that of endothelial cells to blood
flow (Frangos et al. 1985; Furchgott and Vanhoutte 1989; Hecker et al. 1993).
In the vascular system, changes in arterial diameter occur in response to changes
in blood flow rate, in order to ensure a constant vessel tone, and endothelial
cells are widely recognized as the mechanosensory cells of this response. The
early response of endothelial cells to fluid flow in vivo includes the release
of NO and prostaglandins (Hecker et al. 1993). Surprisingly therefore, bone
tissue seems to use a similar sensory mechanism to detect and amplify mechanical
information as the vascular system.
PGE2 upregulation continued throughout the one hour PFF treatment, and also
at least one hour after PFF treatment (Klein-Nulend et al. 1997), suggesting
an auto-amplification mechanism whereby a short-lived stimulus such as mechanical
stress is transduced into a sustained cellular response. A major step in prostaglandin
production is the formation of prostaglandin PGG2 and subsequently PGH2 through
the action of prostaglandin G/H synthase (PGHS or cyclo-oxygenase (COX)) on
arachidonic acid (Smith 1989). There are two distinct enzymes for PGHS, encoded
by separate genes (Kujubu et al. 1991; Rosen et al. 1989). PGHS-1 (or COX-1)
is expressed constitutively in many tissues but can be upregulated by serum
and growth factors (DeWitt 1989). In contrast, the expression of mRNA for PGHS-2
(or COX-2) is not constitutive in most tissues among which bone (Pilbeam et
al. 1993), but can be induced rapidly and transiently by a variety of acute
cell stresses, such as inflammatory mediators (Kujubu et al. 1991) and growth
factors (Pilbeam et al. 1993). We examined the effect of mechanical stress on
expression of PGHS-1 and PGHS-2 in mouse calvarial bone cells. PFF treatment
induced the expression of PGHS-2 within 1 hour (Klein-Nulend et al. 1997). In
the presence of 2% freshly added fetal bovine serum (FBS), which by itself induces
PGHS-2 expression, the stimulating effect of PFF was about 2-fold. When serum
was reduced to 0.1%, the inductive effect of PFF on PGHS-2 was 8 to 9-fold,
relative to static controls. No effect was found on PGHS-1 expression. PFF treatment
also increased the production of PGE2 as well as PGI2 and PGF2a , both acutely
during PFF and for at least one hour after PFF treatment (Klein-Nulend et al.
1997). The enhanced expression of PGHS-2 continued also for at least one hour
after PFF treatment. These results suggest that the mechanical stress had no
effect on PGHS-1, but selectively upregulated PGHS-2 synthesis.
Interestingly, a recent study by Forwood (1996) suggests that induction of PGHS-2
(or COX-2) is important for the induction of adaptive bone formation in vivo.
In that study, rats were treated with a specific PGHS-2 inhibitor (NS-398),
or indomethacin which primarily inhibits PGHS-1, before loading one tibia by
four-point bending (Turner et al. 1994). Endocortical bone formation was significantly
increased 5-8 days after a single bout of loading (300 cycles, 65N) but not
by sham loading. The increase in endocortical bone formation caused by bending
was completely prevented by NS-398, but only partially by indomethacin, even
at very high doses (Forwood 1996). These results suggest that induction of PGHS-2
(or COX-2) is important for lamellar bone formation elicited by mechanical strain.
Therefore, the in vivo induction of PGHS-2 by fluid flow treatment mimics a
critical event in the adaptive response to loading in vivo. This suggests that
fluid flow-treatment of bone cells in vivo is indeed a meaningful way to mimic
the effect of mechanical loading of bone tissue in vivo.
Near weightlessness and the
response of bone cells to mechanical stress
As stated earlier in this proposal, near weightlessness negatively affects the
skeleton and there is evidence that bone cells are directly influenced by micro-g
conditions (Carmeliet et al. 1996; Kumei et al. 1996; Van Loon et al. 1995).
The loss of bone mineral during spaceflight could be solely the effect of an
unusual form of unloading of the skeleton as a result of weightlessness. In
that case countermeasures developed on Earth against disuse osteoporosis should
also be effective against spaceflight-related osteoporosis. However, recent
observations on the non-linear behavior of in vitro preparations of microtubules
(Tabony 1994; Tabony and Job 1990; 1992) suggest an alternative explanation
that seems worthwhile to consider.
Microtubules are an important part of the cytoskeleton, and several observations
on plant- and animal cells indicate that effects of near weightlessness are
likely established via the cytoskeleton (for a review, see Moore and Cogoli
1996). We recently found that the cytoskeleton is involved in the transduction
of the extracellular mechanosignal to the intracellular domain, and in the translation
into prostaglandin signaling (Ajubi et al. 1996). Therefore an alternative explanation
of the interference of near weightlessness with bone cell function may be that
under near weightlessness conditions the mechanosensitivity of bone cells is
impaired. Impaired bone cell mechanosensitivity might subsequently lead to a
negative bone balance, even when countermeasures such as strenuous exercise
are taken by astronauts. The experiments on microtubules assembly (Tabony 1994;
Tabony and Job 1990; 1992) as well as bone cell mechanosensitivity (Ajubi et
al. 1996) were performed on earth and not during spaceflight. It seems worthwhile
to further explore the hypothesis of a direct interaction of near weightlessness
with cytoskeleton-mediated cellular processes such as prostaglandin signaling
in well-controlled studies under near weightlessness conditions. Such studies
will doubtlessly make a significant contribution to furthering our understanding
of the role of gravity in living cells, and could shed new light on the phenomenon
of near weightlessness-related osteopenia.
References
·
Ajubi,
N.E., Klein-Nulend, J., Nijweide, P.J., Vrijheid-Lammers, T., Alblas, M.J.,
and Burger, E.H. Pulsating fluid flow increases prostaglandin production by
cultured chicken osteocytes -a cytoskeleton-dependent process. Biochem Biophys
Res Commun 225:62-68; 1996.
·
Bodine, P.V.N., Trailsmith, M., and Komm, B.S. Development and
characterization of a conditionally transformed adult human osteoblastic cell
line. J. Bone Miner. Res. 11:806-819; 1996.
·
Brillouet,
C. Biorack on Spacelab IML-1. Mattok, C., Ed. SP-1162. ESA Publication Div.
ESTEC, Noorwijk, The Netherlands, 1995.
·
Burger, E.H., and Veldhuijzen, J.P. Influence of mechanical factors
on bone formation, resorption, and growth in vitro. Hall, B.K., Ed. Bone. Vol.
7. Boca Raton, Florida: CRC Press; 1993; 37-56.
·
Cogoli,
A., Friedrich, U., Mesland, D., Demets, R. Life sciences experiments on sounding
rockets (1985-1994). Wilson, A., Ed. SP-1206. ESA Publication Div. ESTEC, Noorwijk,
The Netherlands, 1997.
·
Carmeliet,
G., Nys, G., and Bouillon, R. Differentiation of human osteoblastic cells (MG-63)
in vivo is decreased under microgravity conditions. Proc Sixth Eur Symp
on Life Sciences Res in Space, ESA SP-390:279-282; 1996.
·
Cowin,
S.C., Moss-Salentijn, L., and Moss, M.L. Candidates for the mechanosensory system
in bone. Adv Bioengin 20:313-316; 1991.
·
Demets,
R. Biological experiments on Bion-8 and Bion-9. Burk, W.R., Ed. SP-1190. ESA
Publication Div. ESTEC, Noorwijk, The Netherlands, 1996.
·
De
Witt, D.L. The eicosanoids and their biochemical mechanisms of action. Biochem
J 259:315-324; 1989.
·
Forwood,
M.R. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation
by mechanical loading in vivo. J Bone Miner Res 11:1688-1693; 1996.
·
Frangos,
J.A., Eskin, S.G., McIntire, L.V., and Ives, C.L. Flow effects on prostacyclin
production by cultured human endothelial cells. Science 227:1477-1479; 1985.
·
Furchgott,
R.F., and Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors.
FASEB J 3:2007-2018; 1989.
·
Green, L.C.,
Wagner, D.A., Glogowski, J., Skipper, P.L., Wishnok, J.S., and Tannenbaum, S.R.
Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids.
Anal. Biochem. 126:131-138; 1982.
·
Hecker,
M., Mülsch, A., Bassenge, E., and Busse, R. Vasoconstriction and increased flow:
two principal mechanisms of shear stress-dependent endothelial autacoid release.
Am J Physiol 265 (Heart Cir Physiol 34):H828-H833; 1993.
·
Houde,
J.P., Schulz, L.A., Morgan, W.J., Breen, T., Warhold, L., Crane, G.K., and Baran,
D.T. Bone mineral density changes in the forearm after immobilization. Clin
Orthopaed Rel Res 317:199-205; 1995.
·
Highes-Fulford,
M., Lewis, M.L. Effects of microgravity on osteoblast growth activation. Exp.
Cell Res. 224:103-109; 1996.
·
Klein-Nulend,
J., Van der Plas, A., Semeins, C.M., Ajubi, N.E., Frangos, J.A., Nijweide, P.J.,
and Burger, E.H. Sensitivity of osteocytes to biomechanical stress in vivo.
FASEB J 9:441-445; 1995a.
·
Klein-Nulend,
J., Veldhuijzen, J.P., and Burger, E.H. Increased calcification of growth plate
cartilage as a result of compressive force in vitro. Arthritis Rheum 29:1002-1009;
1986.
·
Klein-Nulend,
J., Semeins, C.M., Ajubi, N.E., Nijweide, P.J., and Burger, E.H. Pulsating fluid
flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal
fibroblasts - correlation with prostaglandin upregulation. Biochem Biophys Res
Commun 217:640-648; 1995b.
·
Klein-Nulend, J., Burger, E.H., Semeins, C.M., Raisz, L.G., and
Pilbeam, C.C. Pulsating fluid flow stimulates prostaglandin release and inducible
prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone
Miner Res; in press; 1997.
·
Klein-Nulend,
J., Helfrich, M.H., Sterck, J.G.H., MacPherson, H., Joldersma, M., Ralston,
S.H., Semeins, C.M., and Burger, E.H. Nitric oxide response to shear stress
by human bone cell cultures is endothelial nitric oxide synthase dependent.
Biochem. Biophys. Res. Commun., in press
·
Kufahl,
R.H., and Saha, S. A theoretical model for stress-generated flow in the canaliculi-lacunae
network in bone tissue. J Biomech 23:171-180; 1990.
·
Kujubu,
D.A., Fletcher, B.S., Varnum, B.C., Lim, R.W., Herschman, H.R. TIS 10, a phorbol
ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin
synthase/cyclooxygenase homologue. J Biol Chem 266:12866-12872; 1991.
·
Kumei,
Y., Shimokawa, H., Katano, H., Hara, E., Akiyama, H., Hirano, M., Mukai, C.,
Nagaoka, S., Whitson, P.A., and Sams, C.F. Microgravity induces prostaglandin
E2 and interleukin-6 production in normal rat osteoblasts: role in
bone demineralization. J Biotechnology 47:313-324; 1996.
·
Mesland,
D.A.M. Novel ground-based facilities for research in the effects of weight.
ESA Microgravity News. Vol 9 (1), pp. 5-10, 1996.
·
Mesland,
D., Brillouet, C., Biorack on Spacelab D-1. Longdon, N., David, V., Ed. SP-1091.
ESA Publication Div. ESTEC, Noorwijk, The Netherlands, 1997.
·
Moore,
D., and Cogoli, A. Gravitational and space biology at the cellular level. Moore,
D., Bie, P., and Oser, H., Eds. Biological and medical research in space. Berlin:
Springer; 1996; 1-106.
·
Piekarski,
K., and Munro, M. Transport mechanism operating between blood supply and osteocytes
in long bones. Nature 269:80-82; 1977.
·
Pilbeam,
C.C., Kawaguchi, H., Hakeda, Y., Voznesensky, O., Alander, C.B., Raisz, L.G.
Differential regulation of inducible and constitutive prostaglandin endoperoxide
synthase in osteoblastic MC3T3-E1 cells. J Biol Chem 268:25643-25649; 1993.
·
Rao,
J., and Otto, W.R. Fluorimetric DNA assay for cell growth estimation. Anal.
Biochem. 207:186-192; 1992.
·
Reich,
K.M., Gay, C.V., and Frangos, J.A. Fluid shear stress as a mediator of osteoblast
cyclic adenosine monophosphate production. J Cell Physiol 143:100-104; 1990.
·
Rosen,
G.D., Birkenmeier, T.M., Raz, A., Holtzman, M.J. Identification of a cycloonxygenase-related
gene and its potential role in prostaglandin formation. Biochem Biophys Res
Commun 164:1358-1365; 1989.
·
Smith,
W.L. The eicosanoids and their biochemical mechanisms of action. Biochem J 259:315-324;
1989.
·
Smith, P.K.,
Krohn, R.I., Hermansom, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D.,
Fujimoto, E.K., Goeke, N.M., Olson, B.J., and Klenk, D.C. Measurement of protein
using bicinchoninic acid. Anal. Biochem. 150: 76-85; 1985
·
Sterck,
J.G.H. Growth and mechanosensitivity of human bone cells - effects of aging
and metabolic bone disease. Academic thesis, Vrije Universiteit, Amsterdam;
1996.
·
Tabony,
J. Morphological bifurcations involving reaction-diffusion processes during
microtubule formation. Science 264:245-248; 1994.
·
Tabony,
J., and Job, D. Spatial structures in microtubular solutions requiring a sustained
energy source. Nature 346:448-451; 1990.
·
Tabony,
J. and Job, D. Gravitational symmetry breaking in microtubular dissipative structures.
Proc Natl Acad Sci USA 89:6948-6952; 1992.
·
Tjandrawinata,
R.R., Vincent, V.L., Hughes-Fulford, M. Vibration force alters mRNA expression
in osteoblasts. FASEB J. 11:493-497; 1997.
·
Turner,
C.H., Forwood, M.R., Rho, J.Y. and Yoshikawa, T. Mechanical loading thresholds
for lamellar and woven bone formation. J Bone Miner Res 9:87-97; 1994.
·
Van
den Bergh L.C., Schelling R., van Ravestijn S.A., Huijser R.H. LIDIA: A new
biological experiment unit for microgravity research. Proc. 6th European Symp.
On Life Science Research in Space. ESA SP-390, 1996.
·
Van
der Plas, A., and Nijweide, P.J. Isolation and purification of osteocytes. J
Bone Miner Res 7:389-396; 1992.
·
Van
Loon J.J.W.A., Veldhuijzen J.P., Windgassen E.J., Brouwer T., Wattel K., Vilsteren
M., van and Maas P. Development of tissue culture techniques and hardware to
study mineralization under microgravity. Adv. Space Res., 14:289-298, 1994.
·
Van
Loon J.J.W.A., van den Bergh L.C., Schelling R., Veldhuijzen J.P., Huijser R.H..
‘Development of a centrifuge for acceleration research in cell and developmental
biology’. 44th International Astronautical Congress, IAF/IAA-93-G.4.166, Gratz,
Austria, October 1993.
·
Van
Loon, J.J.W.A., Bervoets, D.J., Burger, E.H., DieudonnJ, S.C., Hagen, J.W.,
Semeins, C.M., Zandieh Doulabi, B., and Veldhuijzen, J.P. Decreased mineralization
and increased calcium release in isolated fetal mouse long bones under near
weightlessness. J Bone Miner Res 10:550-557; 1995.
·
Van Loon, J.J.W.A., Veldhuijzen, J.P., and Burger, E.H. Bone and
space flight: an overview. Moore, D., Bie, P., and Oser, H., Eds. Biological
and medical research in space. Berlin: Springer; 1996; 259-299.
·
Weinbaum,
S., Cowin, S.C., and Zeng, Y. A model for the excitation of osteocytes by mechanical
loading-induced bone fluid shear stressses. J Biomech 27:339-360; 1994.
Acknowledgement
Space Research Organisation of the Netherlands (SRON) grant MG-055 (FlowSpace)
and MG-057 (DESC).